The product of a joint development programme between by Elan Pharmaceuticals and Biogen, Tysabri (natalizumab) is a humanised therapeutic monoclonal antibody (MAb) and the first alpha-4 antagonist in a new class of agents called selective adhesion-molecule (SAM) inhibitors. Tysabri, previously known as Antegren, is an innovative treatment for multiple sclerosis (MS) and Crohn’s disease.
Hailed by analysts as a breakthrough in the treatment of MS when launched in the US at the end of 2004, Tysabri’s market debut proved short-lived. At the end of February 2005, the drug was voluntarily withdrawn after three patients developed progressive multifocal leukoencephalopathy (PML), a rare but often fatal nervous system disorder.
However, in July 2006, the US FDA approved the reintroduction of Tysabri as monotherapy for relapsing MS to slow the progression of disability and reduce the frequency of clinical relapse.
Tysabri now has a revised label with enhanced safety warnings and in the US can only be administered under the TOUCH Prescribing Program. Europe followed suit and allowed the reintroduction of Tysabri for relapsing-remitting MS.
Although the EMEA has taken a negative view of Tysabri as a treatment for Crohn’s disease, 2008 saw its approval by the FDA. In the US it is indicated as a treatment for moderate-to-severe Crohn’s disease in patients with evidence of inflammation and who have had an inadequate response to, or are unable to tolerate, conventional therapies for Crohn’s disease.
Consistent with its use in MS, Crohn’s disease patients receiving Tysabri must also be enrolled in a special restricted distribution programme. This is called CD TOUCH (the Crohn’s Disease–Tysabri Outreach Unified Commitment to Health Prescribing Program).
Safety issues continue to haunt Tysabri with recent warnings from regulators in both the US and Europe of a potentially higher risk of liver damage (elevated hepatic enzymes and bilirubin) among patients taking the drug. As a consequence, patients taking Tysabri are required to have regular liver function tests and treatment discontinued in any patient judged at risk.
.
SAM inhibitors represent a new class of therapeutic agents
Tysabri (natalizumab) is a SAM inhibitor that works by binding to cell surface receptors known as alpha-4-beta-1 (VLA-4) and alpha-4-beta-7 integrins. Alpha-4 integrins are important in mediating the migration of leukocytes (white blood cells) from the bloodstream to sites of inflammation in the tissues.
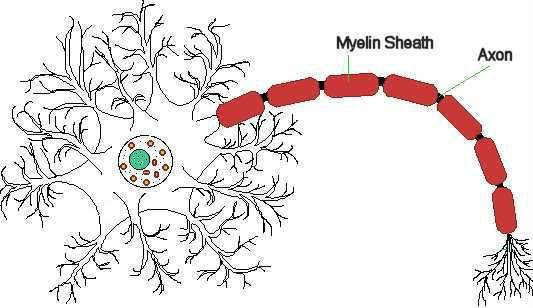
By binding to alpha-4 integrin receptor sites, Tysabri selectively blocks leukocyte adhesion to blood vessel walls and subsequent migration of these immune cells to sites of chronic inflammation, where they can exacerbate the inflammatory process. Leukocytes are believed to mediate the damage to the protective myelin sheath of nerve cells seen in multiple sclerosis.
Expanding treatment options for patients with multiple sclerosis
Estimates suggest that worldwide around 2.5 million people have multiple sclerosis, an inflammatory disease of the nervous system characterised by recurrent relapses followed by periods of remission. After trauma, it is the second most common neurological disability to affect young and middle-aged adults.
The clinical efficacy of Tysabri in MS is supported by data from a number of clinical trials, including the pivotal phase III AFFIRM (natalizumab safety and efficacy in relapsing-remitting MS) trial that was designed to assess the ability of Tysabri to slow the progression of disability (primary endpoint) as well as reduce the rate of clinical relapse.
Results from the AFFIRM trial showed that over two years, treatment with Tysabri reduced the risk of disability progression by 42% (p<0.001), while also producing a 67% reduction in the annualised relapse rate compared to placebo (p<0.001). Sustained and statistically significant reductions in brain lesion activity as measured by MRI were also seen in the Tysabri treatment arm.
The second pivotal phase III trial, SENTINEL (safety and efficacy of natalizumab in combination with Interferon beta-1a), evaluated the efficacy of Tysabri in combination with Biogen’s Avonex (interferon beta-1a). Results over two years showed that the addition of Tysabri to ongoing interferon beta-1a therapy had a significant effect on disability progression, relapse rate and brain MRI disease activity compared to interferon beta-1a therapy alone.
Mixed results from clinical trials in Crohn’s disease
In contrast to trials in patients with MS, results from the clinical trials of Tysabri (natalizumab) in Crohn’s disease patients have proved equivocal. While early trials suggested that Tysabri was effective in inducing remission in patients with active Crohn’s disease, this was not supported by data from the phase III ENACT-1 trial which failed to meet its primary efficacy endpoint. However, more encouraging data have since emerged from the ENACT-2 trial, which suggest that Tysabri is effective in maintaining remission once an acute flare-up has been brought under control.
Results from the two ENACT trials presented the companies with a dilemma as the data suggested that while not effective in inducing remission in patients with active disease, Tysabri was nonetheless effective in maintaining remission. Following discussion with the FDA an additional induction trial was planned. Results of this phase III trial, called ENCORE, proved encouraging.
In patients with moderate to severe Crohn’s disease and active inflammation, treatment with Tysabri met the primary endpoint (a 70-point decrease in baseline CDAI score at eight and 12 weeks). All trial secondary endpoints, which included clinical remission (CDAI score ≤ 150) at both eight and 12 weeks, were also met.
Marketing commentary
MS is a chronic and disabling disease, with healthcare costs disproportionate to the numbers affected. In the US alone, costs are estimated to exceed $10bn a year. The introduction of interferon beta 1a revolutionised treatment for many patients with MS and is reflected in sales of Biogen’s Avonex, current US market leader.
Biogen and Elan Pharmaceutical continue to have high hopes for Tysabri, despite setbacks over the drug’s safety profile. They received a boost in July 2007 when the UK National Institute for Health and Clinical Excellence (NICE) recommended use of Tysabri for patients with highly active relapsing remitting MS, a particularly debilitating form of the disease. In addition, Tysabri has now been approved for Crohn’s disease in the US, expanding its label.