The product of a joint development programme between Genentech and Roche, Avastin (bevacizumab) is a humanised monoclonal antibody (MAb) directed against vascular endothelial growth factor (VEGF), a pro-angiogenesis factor.
Avastin broke new ground in becoming the first angiogenesis inhibitor to market, when it secured US FDA approval at the end of February 2004 and in Europe in early 2005, for use as a first-line treatment for patients with metastatic colorectal cancer. Since then it has been approved as a treatment for breast and lung cancer, where it has similarly demonstrated a progression-free or overall survival benefit.
In April 2009 Avastin failed a major study (C-08) to see its impact on the recurrence of colon cancer in patients after they had surgery. Seen as one of the key reasons behind Roche’s purchase of Genentech, the news of the drug’s ineffectiveness in this case sent shares of Roche tumbling. Annual sales of Avastin have soared to $4.4bn and analysts had predicted this figure could double if the drug was found to delay progression of the disease in early-stage patients.
However, Avastin in combination with paclitexal chemotherapy has been found effective for the first-line treatment of HER2-negative breast cancer. In February 2008, the drug was approved for the respective use by the FDA.
Genentech applied for a supplemental biologics license to the FDA for approving Avastin’s use in treating first-line metastatic renal cell carcinoma. The drug received full approval from the FDA for this indication in August 2009. Studies are also progressing to assess its potential use in other tumour types with different chemotherapy regimens and other targeted therapies like Dulanermin, Apomab, Herceptin, Tarceva and so on.
Avastin was approved by the FDA for the treatment of recurrent glioblastoma in 2009. However, it was rejected for the same indication by the European Medicines Agency (EMA) in 2010.
European approval was also granted for the use of Avastin in combination with interferon for the treatment of renal cell carcinoma, for which a significant improvement in progression-free survival was observed.
Roche submitted a marketing authorisation application to the EMA to use the drug to treat ovarian cancer in February 2011. The EMA approved the drug in December 2011 as a major treatment for women newly diagnosed with advanced ovarian cancer. The drug in combination with chemotherapy (carboplatin and gemcitabine) also received positive opinion from the EU, to treat women with recurrent, platinum-sensitive ovarian cancer in September 2012 and approval was granted in October 2012.
In November 2011, FDA rejected the use of Avastin for the treatment of metastatic breast cancer, stating that it was not safe or effective. In the same month, Avastin in combination with paclitaxel chemotherapy obtained European approval for women with metastatic breast cancer in the first-line setting.
The FDA approved Avastin in combination with fluoropyrimidine-based irinotecan or oxaliplatin chemotherapy for people with metastatic colorectal cancer (mCRC) in January 2013. The combination works as a second line treatment for the people who received Avastin plus an irinotecan or oxaliplatin containing chemotherapy as first-line treatment for mCRC.
Avastin in combination with radiotherapy and temozolomide chemotherapy got approval from the Japanese Ministry of Health, Labour and Welfare (MHLW) in June 2013 for the treatment of malignant glioma, including newly diagnosed glioblastoma (GBM). It was also approved as a monotherapy for the treatment of recurrent gliblastoma and for some other types of high grade glioma.
The US FDA approved Avastin to treat patients with persistent, recurrent or late-stage (metastatic) cervical cancer in August 2014.
Further promising clinical data continue to be released. In metastatic colorectal cancer, a median overall survival benefit of approximately two years was achieved when Avastin was used in combination with standard chemotherapy. Patients on this regimen who had surgery to remove the tumour had double the chance of surviving at two years compared to those who did not (89% vs 44% respectively).
In addition, Avastin has emerged as the only biological therapy to confer overall survival benefit when used first-line in combination with chemotherapy in patients with K-Ras wild-type metastatic colorectal cancer.
Meanwhile, new data from the AVAiL trial has shown that Avastin significantly slows disease progression in advanced non-small cell lung cancer (NSCLC), with some patients surviving for over a year.
Avastin at competitive disadvantage in UK
The UK National Institute of Clinical Excellence (NICE) issues guidance on the use of drugs every year. The institute released its guidance on the use of Avastin, Sunitinib, sorafenib and temsirolimus for renal cell carcinoma in early 2009. Avastin was at an obvious competitive disadvantage when the final guidance issued by NICE in March 2009 recommended Pfizer’s Sunitinib for the first-line treatment of renal cancer.
NICE also suspended the recommendation of bevacizumab for use in lung cancer when Genentech did not submit evidence for the appraisal earlier in 2007–2008.
Further, in 2007, Avastin lost ground when NICE turned down the use of bevacizumab in combination with 5-fluorouracil plus folinic acid, with or without irinotecan for curing metastatic colorectal cancer in people never treated previously. All of these developments have put Genentech’s drug at a disadvantage over its competitors.
Avastin blocks VEGF receptor binding
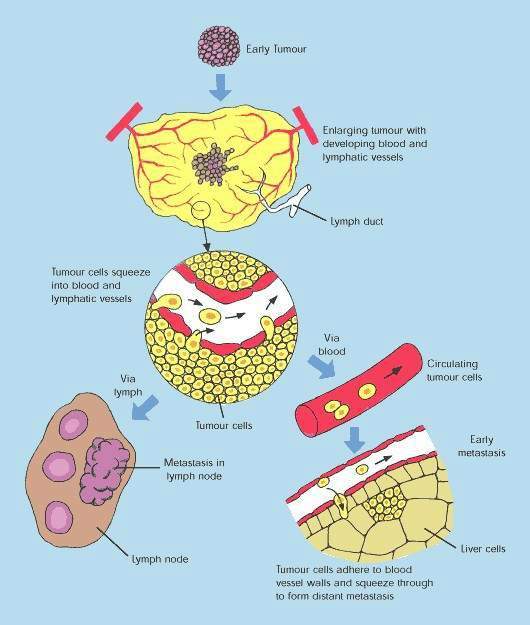
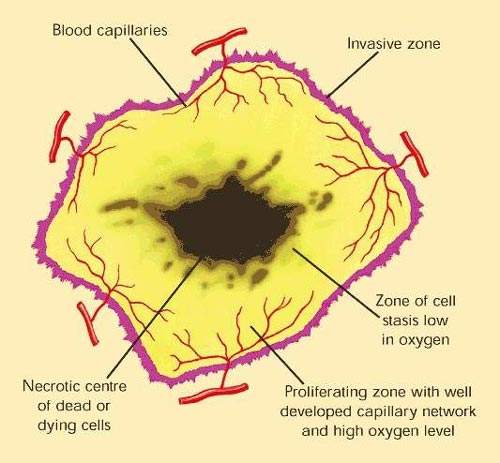
Angiogenesis inhibitors are drugs which are designed to stop tumours from developing a blood supply, a pre-requisite for tumour growth and metastasis (tumour spreading).
Avastin works by inhibiting the action of VEGF, a specific angiogenesis growth factor that binds to receptors on blood vessels and stimulates the formation of new blood vessels. By binding to VEGF, Avastin blocks VEGF receptor binding.
The idea of attacking tumours by cutting off their blood supply was first described in the early 1970s. It has been hailed as one of the most exciting approaches to the treatment of solid tumours.
Over 50 angiogenesis inhibitors are in development for cancer, reflecting the level of interest in this new class of anti-cancer agents.
These findings suggest that the addition of Avastin to standard chemotherapy as first-line therapy confers survival benefit in patients with advanced breast cancer. Avastin was generally well tolerated by patients in this study, where it was administered at the recommended dose of 10mg/kg every two weeks.
Survival benefit in lung cancer patients
After colorectal and breast cancer, lung cancer was the third major cancer in which Avastin demonstrated a survival benefit. Evidence for its efficacy in one of the most difficult-to-treat cancers was seen in results from the pivotal E4599 trial.
Involving 878 patients with locally advanced, metastatic or recurrent NSCLC, the study showed that patients receiving Avastin plus chemotherapy (paclitaxel and caroboplatin) had a 25% reduction in risk of death compared with those receiving chemotherapy alone. Median survival was 12.3 months in the Avastin treatment arm, compared with 10.3 months in the control arm.
Improvement in overall survival among Avastin-treated patients was accompanied by improvement in progression-free survival (35% reduction in risk of progression) when compared with chemotherapy alone. More patients in the Avastin treatment arm also responded to therapy compared with those on chemotherapy alone (29% vs. 13% respectively).
Positive results also emerged from the recently completed European AVAiL trial. In this phase III trial involving over 1,000 patients with previously untreated advanced NSCLC, two doses of Avastin in combination with gemcitabine and cisplatin were compared with platinum-based therapy alone. Again, results showed a significant improvement in progression-free survival in the Avastin treatment arm as well as higher and more durable response rates.
Survival benefit in kidney cancer patients
Evidence is accumulating to suggest that the survival benefit first seen in patients with metastatic colorectal cancer also extends to patients with other solid tumours, most notably breast and lung cancer and, now, kidney cancer.
Trials in patients with renal cell carcinoma, in which Avastin was added to interferon, suggest that it can make a significant improvement in progression-free survival.
In the randomised, double-blind, placebo-controlled AVOREN trial, the addition of Avastin to interferon in patients with advanced kidney cancer resulted in a significantly longer median duration of progression-free survival compared with interferon alone (10.2 months vs. 5.4 months; HR 0.63, 95% CI 0.52–0.75; p=0.0001).
Patients who had received previous systemic treatment for metastatic renal cell carcinoma were excluded from the study, thus all patients were treatment naïve at study entry.
Until relatively recently, there were few treatment options for patients with renal cell carcinoma. Now four new drugs are available for renal cell carcinoma, which in addition to Avastin, include sunitinib, sorafenib and temsirolimus. It is anticipated that future studies will likely compare Avastin as single-agent, first-line therapy with these new therapies as well as in combination with these new agents.
Survival benefit in ovarian cancer patients
Phase III clinical trials in patients with metastatic ovarian cancer included the GOG 0218 and ICON 7 studies. The GOG 0218 trial recruited around 1,873 patients with untreated ovarian cancer, primary peritoneal or fallopian tube carcinoma. Around 1,528 patients were enrolled for III ICON7 trial. Delayed progression was observed in both.
The most recent Phase III trial, the OCEANS study, was conducted in 484 patients with platinum-sensitive recurrent ovarian cancer. The study included two patient groups treated with Avastin in combination with carboplatin and gemcitabine and Avastin alone or a placebo in combination with carboplatin and gemcitabine followed by placebo alone. Results released in February 2011 indicated that women treated with Avastin in combination with carboplatin and gemcitabine showed delayed progression when compared to those treated with placebo.
Marketing commentary
Early enthusiasm for angiogenesis inhibitors waned after several promising drugs failed to meet increased survival endpoints in pivotal phase III trials in cancer patients. The survival benefit first seen with Avastin in the colorectal cancer trials was important in demonstrating proof of principle, as well as increasing treatment options for patients with metastatic colorectal cancer.
Avastin is now seen as having the potential to be incorporated into a range of treatment regimens for patients with a variety of solid tumours, including colorectal, breast, lung and kidney cancer.