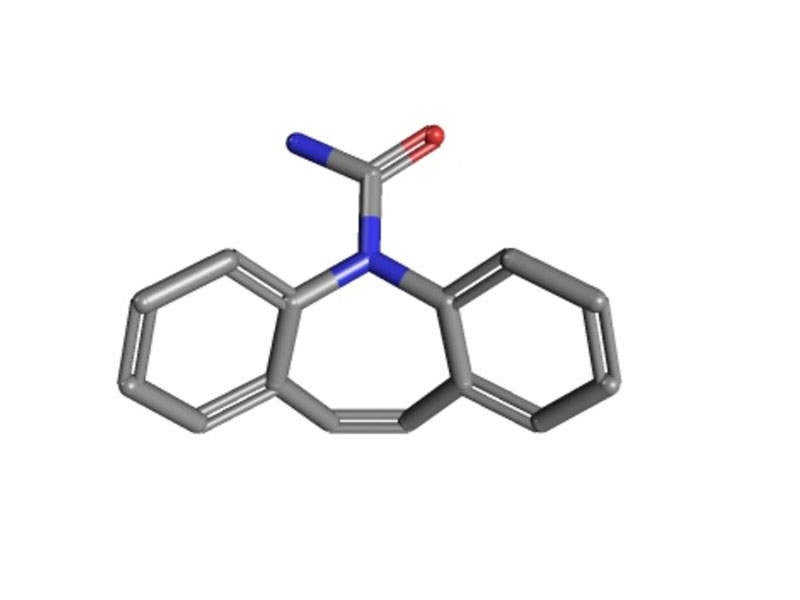
Carnexiv (carbamazepine) is an injectable formulation developed by Lundbeck for partial, generalised tonic-clonic seizures in adult patients who are unable to temporarily take oral medication.
The US Food and Drug Administration (FDA) granted orphan drug designation for Carnexiv in 2013 and Lundbeck resubmitted the new drug application (NDA) for the drug on 22 April 2016 in reply to the complete response letter to the FDA. Carnexiv was then approved by the FDA as a short-term replacement therapy for certain types of seizures on 7 October 2016.
Lundbeck developed carbamazepine using Ligand Pharmaceutical’s captisol technology. Ligand Pharma has received a milestone payment of $1.25m from Lundbeck following the approval of Carnexiv and Ligand will also receive a royalty of 2.75% on the net sales of the drug.
Seizure types and symptoms
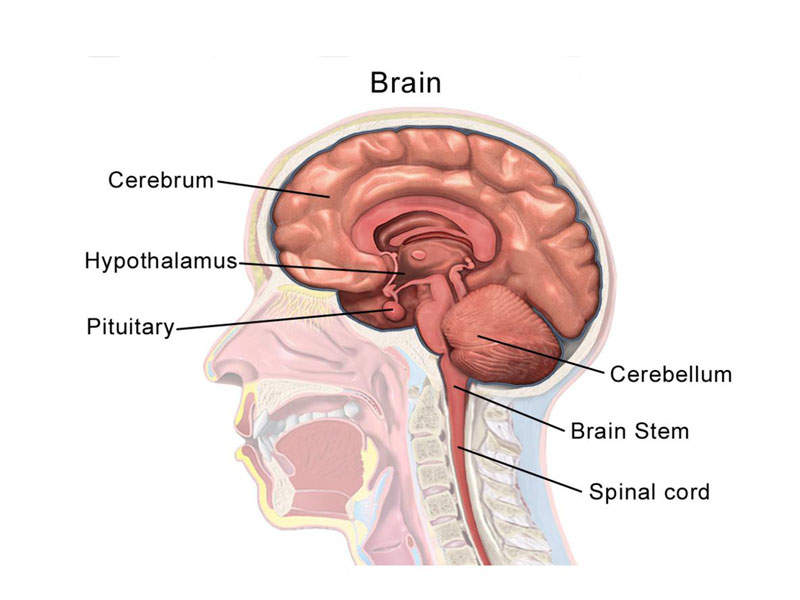
A seizure is a physical behaviour, which occurs due to abnormal electrical activity in the brain. It causes uncontrollable contractions and relaxations of muscles, as well as rapid and rhythmic shaking of the body.
There are two types of seizure, partial (focal), generalised tonic-clonic and mixed pattern. There are also two types of partial seizure, simple and complex. A simple partial seizure affects a small region of the brain. Patient’s memory or awareness are not affected and they retain consciousness.
A complex partial seizure is an epileptic seizure that affects the unilateral cerebral hemisphere. It is usually associated with loss of awareness, memory, and impaired consciousness.
Generalised tonic-clonic seizure affects the whole brain and involves the entire body. It can occur in a single episode or repeatedly as chronic illness called epilepsy. Symptoms include biting the cheek or tongue, difficulty in breathing, blue skin colour, and loss of control over urine or stool.
Mixed pattern seizures are characterised by symptoms of both partial and generalised seizures.
Carnexiv’s mechanism of action
Carnexiv is an anti-epileptic drug available in single-dose 20ml vials containing 200mg of carbamazepine. It is approved as a short-term replacement therapy for oral carbamazepine.
The exact mechanism of action of the drug is unknown but the principle component carbamazepine, carbamazepine-10, 11-epoxide is known to exert anti-convulsant activity.
Clinical trials on Carnexiv
The US FDA approved Carnexiv based on the results obtained from OV-1015 and 1318A clinical trials, which were conducted to evaluate the safety and efficacy of the drug.
OV-1015 was a phase I, multi-centre, sequential, open-label study conducted to examine the safety and tolerability of intravenous carbamazepine in comparison with oral carbamazepine.
The trial enrolled 98 adult subjects with epilepsy, who either received 10mg/ml carbamazepine dissolved in 250mg/ml of Captisol (cyclodextrin) administered intravenously every six hours or 400-800mg a day of oral carbamazepine.
The trial demonstrated that the intravenous administration of carbamazepine was well tolerated by the subjects as a short-term treatment.
The 1318A trial was a phase III, multi-centre, open-label trial conducted to evaluate the safety and efficacy of the intravenously administered Carnexiv in adult patients with epilepsy. The trial enrolled 108 epilepsy patients, who are already under treatment with oral carbamazepine.
The trial included a 28-day lead in period where patients were asked to discontinue oral carbamazepine and receive intravenous administration of carbamazepine for every six hours from day 1 through to day 4.
It demonstrated that intravenous carbamazepine was well tolerated as a short-term replacement for oral carabamazepine. Seizure control was the same when switching between the methods of administration and formulation.