Edarbi (Azilsartan medoxomil) was developed by Takeda Pharmaceuticals for the treatment of hypertension in adults.
Takeda submitted the new drug application (NDA) for Edarbi to the FDA in April 2010. The company conducted seven phase III clinical trials on the drug before submitting the NDA. All the phase III clinical trials met the primary endpoint and showed the statistical superiority of Edarbi when compared to placebo and two other active comparators.
On 26 February 2011, Takeda received the US FDA approval for Edarbi for the treatment of hypertension in adults.
Hypertension
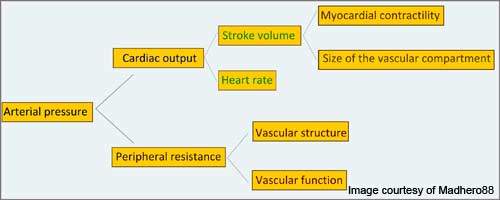
Hypertension is a condition in which the arterial blood pressure increases to 140mm Hg or greater systolic or 90mm Hg or greater diastolic.
Hypertension affects more than 75 million people in the US. It is estimated that every one in three adults suffers from the disease. According to current estimates, about 1 billion people are affected by hypertension worldwide and the same is estimated to increase to 1.5 billion by 2025.
Edarbi mechanism of action
Edarbi acts by blocking a hormone called Angiotensin II that increases blood pressure by constricting the blood vessels. It therefore belongs to the class of drugs known as Angiotensin II receptor blockers (ARB).
The drug is approved to be used in 80mg dose once-daily as oral therapy. It is also available in 40mg dose, which is appropriate for the patients who use diuretics.
Clinical trials
Takeda conducted double-blind, randomised and placebo-controlled phase II clinical trials on Edarbi between May 2006 and December 2006. The purpose of the study was to test the safety, efficacy and tolerability of Edarbi once-daily for eight weeks. The study enrolled 449 patients with hypertension. The primary outcome measure was finding the change in blood pressure from the baseline after eight weeks. The secondary outcome measures included finding the change in both systolic and diastolic blood pressures from the baseline after this period.
The FDA approval for Edarbi was based on seven phase III clinical trials. The clinical studies included four trials with active comparators and five with placebo. The duration of the studies ranged between six weeks and six months. The double-blind and randomised studies enrolled over 5,941 patients with mild, moderate and severe hypertension. Edarbi was administered to 3,672 patients, while 801 patients were administered with placebo and 1,468 patients were given active comparators. Two of the studies compared 40mg and 80mg doses of Edarbi with placebo and active comparators for six weeks to observe the drug’s effect on blood pressure reduction.
Edarbi met the primary endpoint in all the seven trials. Edarbi 40mg/day decreased the 24-hour mean SBP (systolic blood pressure) by 13.2mm Hg from the baseline and Edarbi 80mg/day reduced the SBP by 14.3mm Hg. The blood pressure reductions of 80mm/mg were proved to be statistically superior when compared to the FDA-approved active comparators such as olmesartan medoxomil (Benicar) 40mg/day, valsartan (Diovan) 320mg/day and placebo.
The common side effects associated with Edarbi during the clinical studies were diarrhoea, nausea, asthenia, fatigue, muscle spasm, dizziness and cough.
Takeda initiated phase IV randomised, double-blind, placebo-controlled and parallel group clinical study on Edarbi in March 2011. The study enrolled 70 patients in the US. The primary outcome measure of the study is to find the change in renal plasma. The secondary outcome measure includes finding the change in endothelial function.
Marketing commentary
Prior to approving Edarbi, the FDA approved two drugs for treating hypertension. The two drugs were Benicar which was developed by Sankyo Pharma and Diovan developed by Novartis. Takeda holds the marketing rights of Edarbi in the US.