Elotuzumab (HuLuc63) is an anti-CS1 humanised monoclonal antibody (MAb) developed by PDL Biopharma. In December 2008 PDL Biopharma transferred its biotechnology operations to wholly-owned subsidiary Facet Biotech Corporation, which was spun off the same month. In April 2010, Facet Biotech was acquired by Abbott.
The drug is being used as a treatment for multiple myeloma. Positive findings in preclinical studies have seen elotuzumab advance to clinical development.
Drug therapies for multiple myeloma
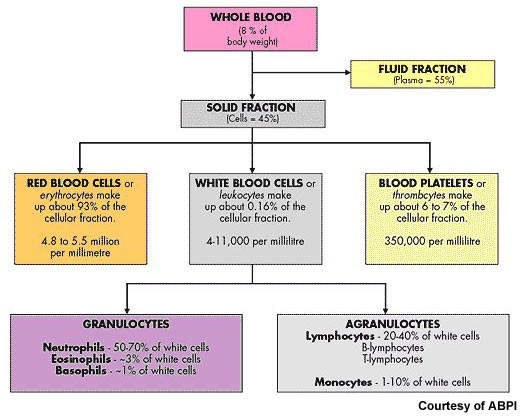
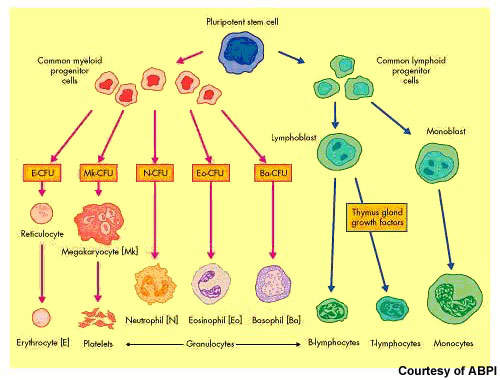
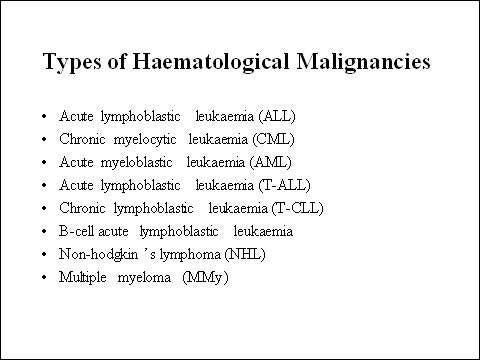
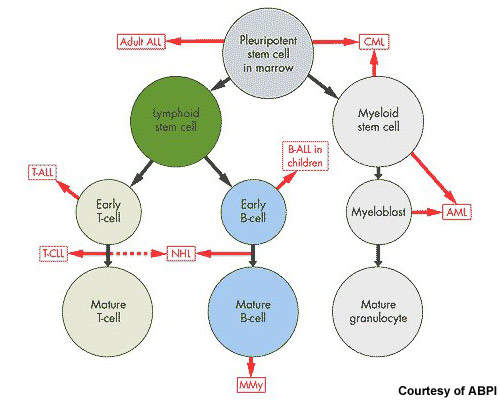
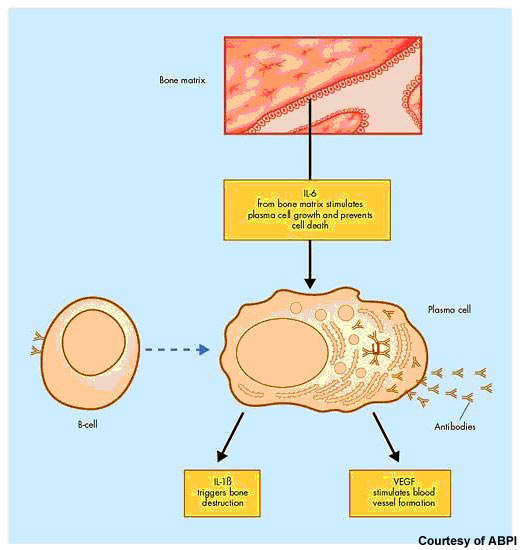
After non-Hodgkin’s lymphoma, multiple myeloma is the second most common haematological malignancy. It arises from the over production of antibody-producing plasma cells leading to the formation of tumours in the bone marrow. As these abnormal malignant cells spread throughout the bone marrow, they disrupt normal production of red blood cells, platelets and white cells.
For decades, cytotoxic chemotherapy has been the mainstay of treatment of multiple myeloma in symptomatic patients, usually augmented with steroids. Melphalan, vincristine, cyclophosphamide, doxorubicin, idrarubicin and carmustine are some of the most commonly used cytotoxic drugs.
More recently, a number of newly introduced biological therapies have expanded treatment options, especially for patients who are refractory to conventional chemotherapy and whose disease has progressed.
These newer therapies include interferon, thalidomide, and lenalinomide, in addition to bortezomib, the first-in-class proteasome inhibitor.
Although there have been some important advances in the treatment of multiple myeloma with the advent of biological therapies, it remains a disease for which new treatments are still urgently needed.
This is highlighted by average five-year survival rates of about 30% and ten-year survival rates of only 3%.
CS1 – a novel target for multiple myeloma
Elotuzumab is directed against CS1, a cell surface glycoprotein that appears highly expressed on myeloma cells but minimally expressed on normal cells. It represents an entirely new therapeutic target with the potential to provide a new form of treatment for multiple myeloma patients.
Preclinical in vitro studies have shown that in the presence of elotuzumab, myeloma cells undergo lysis and death. Researchers suggest that elotuzumab may exert its anti-tumour effects via antibody-dependent cell cytotoxicity in which natural killer (NK) cells play an important role.
Clinical trials
In 2008, three Phase I/II safety and tolerability studies of elotuzumab were initiated in patients with relapsed multiple myeloma. One study was initiated as a monotherapy, the second was in combination with velcade and the third was in combination with revlimid (lenalidomide).
Phase I clinical trials in combination with velcade in relapsed and refractory myeloma patients started in October 2007.
In December 2009, Facet Biotech presented potentially promising data from the third Phase I study, which tested elotuzumab in combination with revlimid. The preliminary data showed that if elotuzumab was administered in combination with revlimid, it might provide a potential treatment option for patients with multiple myeloma.
Out of 28 patients who were administered elotuzumab, 82% achieved an objective response rate. Two patients in the elotuzumab administered group experienced adverse events.
The Phase Ib/II trial was initiated in April 2008 and enrolled around 102 patients with relapsed multiple myeloma. Patients were given elotuzumab along with lenalidomide and dexamethasone. Interim data released in December 2010 showed that dose-limiting toxicities had not observed. The study is expected to be completed by September 2011.
Phase I clinical trials will be initiated in Japan in 2011 and will recruit 20 people with relapsed or refractory multiple myeloma. Patients will be administered elotuzumab along with lenalidomide or a low-dose dexamethasone. The trial is expected to be completed by August 2013.
In January 2010, Facet Biotech announced that the company had enrolled the first patient in a randomised phase II study of elotuzumab in combination with revlimid. The study recruited 98 relapsed myeloma patients over the age of 62 years. Results released in June 2011 indicated that elotuzumab is safe and effective when administered in combination with revlimid.
Enrolment for two Phase III clinical trials on elotuzumab commenced in the first half of 2011.
A Phase III trial for evaluating the safety and efficacy of lenalidomide/dexamethasone with or without elotuzumab in relapsed multiple myeloma patients will enrol 640 patients. The study will begin in 2011 and will evaluate the objective response rates and the overall survival of the patients. The trial includes two patient arms – one will be given lenalidomide with dexamethasone and the other lenalidomide, dexamethasone and elotuzumab. The primary data is expected to be released by March 2014 and the trial is expected to be completed by October 2017.
Another Phase III trial is being conducted in previously untreated myeloma patients. Two patient groups are being administered lenalidomide and dexamethasone with or without elotuzumab. The primary endpoint is progression-free survival. The study began in June 2011 and is expected to be completed by May 2018.
BMS to co-develop elotuzumab
In August 2008, PDL Biopharma signed a co-development and marketing agreement with Bristol-Myers Squibb, following which BMS obtained the rights to lead the global development activities for elotuzumab, while PDL Biopharma focused on completion of the Phase I programme and the support of Phase II studies.
BMS is a leading player in the market for anti-cancer drugs. Its portfolio of anti-cancer drugs includes Erbitux (cetuximab), a therapeutic MAb that is approved for the treatment of metastatic colorectal cancer and locally advanced squamous cell carcinoma of the head and neck.
In January 2010, Facet Biotech was awarded $15m milestone payment by Bristol-Myers Squibb.
Marketing commentary
Despite an expanded range of treatment options for patients with multiple myeloma, there is still urgent unmet need for drugs to improve survival rates. Biological therapies are seen as an important new avenue of research with the potential to generate completely new therapeutic targets for haematological malignancies.
Elotuzumab represents one such example and its progress through clinical trials in multiple myeloma patients will be watched with interest.