Alzheimer's disease (AD) is the most common type of dementia, which severely impacts the intellectual abilities of a patient to carry on with their daily lives.
It is estimated that about 5.3 million individuals in the US are affected with the disease and is the seventh biggest cause of death in the country. To combat the disease, efforts are ongoing to treat and prevent its early onset. Suitable ways to clinically diagnose AD have not been able to yield positive results.
In 2006, it was estimated that more than 26 million individuals across the world were affected with AD. By 2050, this number is expected to increase to more than 100 million. There is a growing need to find an easy, non-invasive imaging method that can predict and diagnose various forms of dementia, particularly AD.
Bayer's florbetaben is one of the first drugs being developed to address this need. It is touted to be a revolutionary new drug that will help in the early detection and diagnosis of AD.
Florbetaben is in Phase III clinical trials, which are not expected to be finalised before 2014. If approved, the drug will not only provide the much-needed early diagnosis of AD but also generate millions in revenue for Bayer. The company is expecting €500m in sales for the drug.
Alzheimer's disease
AD is a fatal and progressive brain disorder that damages the brain cells, leading to memory loss. It causes trouble with thinking and behaviour, which can affect a person's work and social life. The disease is more common in older people than younger people. It usually begins beyond the age of 60 and risk increases with age.
Researchers believe that genetics plays a key role in an individual's risk of developing AD. The disease was first discovered by German physician Alois Alzheimer in 1906 and therefore named after him.
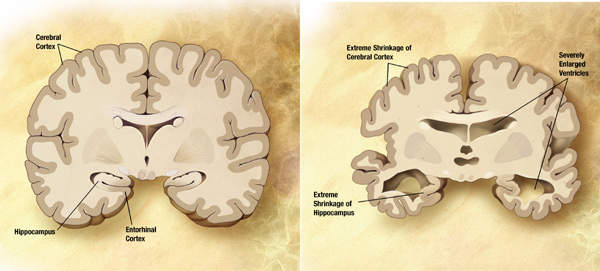
The brain consists of 100 billion nerve cells that communicate with each other to form networks. Each nerve cell network has a particular responsibility such as thinking, learning, hearing and smelling. In AD, many of these brain cells decline and die.
Two abnormal structures known as plaques and tangles are believed to be responsible for damaging and destroying nerve cells. Although people develop few plaques and tangles as they grow old, the number of plaques and tangles in patients suffering from Alzheimer's is far greater.
Plaques develop between nerve cells and contain a protein fragment known as beta-amyloid. Tangles are made of twisted fibres of a protein called tau and develop inside dying cells. Scientists believe that plaques and tangles block communication between nerve cells in some way and interrupt actions that cells need to survive. Alzheimer's has no cure but certain symptoms can be treated. Along with proper support and services some treatments can enable patients to lead a better life.
Florbetaben PET tracer
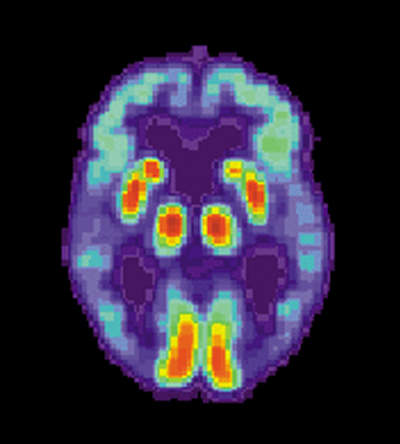
Bayer's new drug florbetaben is an 18F-radiolabelled tracer. Florbetaben binds with beta-amyloid plaques and can be detected using positron emission tomography (PET). PET is a nuclear imaging method that generates a three-dimensional image of functional activities in the body. Florbetaben helps detects beta-amyloid plaques, the main cause of AD.
Diagnostic methods such as cognitive tests, magnetic resonance imaging (MRI) and computerised tomography (CT) scans indicate the probability of having the disease. However, such clinical diagnoses are often made too late. The best way to diagnose AD is through a post-mortem autopsy or a brain tissue biopsy.
A diagnostic tool that can detect the disease in the early stages, before the symptoms are too advanced is needed so that treatment can be started early. Bayer's Florbetaben is expected to address these concerns.
Positive clinical trials and collaboration
In July 2009, Bayer announced positive results from its global Phase II clinical trials. The Phase II trial was an open-label, non randomised, multi-centre study conducted across 18 locations in four countries including Australia, Germany, the US and Switzerland. The study included 213 patients aged 55 years or older and evaluated the efficacy of florbetaben in separating patients with probable AD and healthy volunteers.
About 150 of the 213 individuals in the study received a single intravenous injection of the drug. A 20-minute scan in the phase II trial produced consistent, high-quality images from all the study centers. PET images generated using florbetaben had a specificity of about 90% which means that scans indicated presence of cerebral beta-Amyloid plaques in about 10% of the healthy volunteers.
In October 2009, Bayer announced the company's collaboration with AC Immune. Bayer will provide florbetaben to AC Immune for use in a clinical trial in the sphere of AD. The study is being conducted to develop a therapy alternative for the treatment of AD.
AC Immune will use florbetaben for imaging of beta-amyloid deposition in patients in Phase I clinical trials of the company's Alzheimer's vaccine ACI-24. The vaccine is an active agent which stimulates a patient's immune system to generate beta-sheet conformation-specific antibodies that stop plaque deposition or improve its clearance.
In November 2009, Bayer announced the enrollment of the first patients in the Phase III trial. The open-label, multi-centre, non-randomised single-dose trial will assess the efficacy of florbetaben in the detection of beta-amyloid deposition in the brain.
The trial will enrol 400 individuals with and without manifest dementia. This will include patients with either a high prospect of cerebral beta-amyloid deposition or a low prospect of cerebral beta-amyloid deposition. The expected completion of the trial is in 2011 although it is not expected to be finalised until 2014.