AstraZeneca’s, Galida (tesaglitazar) is a dual-acting oral Peroxisome Proliferator-Activated Receptor (PPAR) agonist that was under development for the treatment of glucose and lipid abnormalities associated with type 2 diabetes and the metabolic syndrome.
Having successfully completed early clinical development, Galida entered phase III clinical trials in late 2003. However, in May 2006 the company decided to abandon any further development of the drug, concluding from trial data that it didn’t offer significant advantages over existing therapies.
Safety has been a major concern with PPAR agonists and had led the US FDA to request additional safety studies as part of a worldwide regulatory review of the safety and tolerability of this new class of drugs.
DUAL PPAR AGONISTS FOR TREATING METABOLIC SYNDROME
Metabolic syndrome, or insulin resistance syndrome as it is sometimes called, is an asymptomatic condition characterised by a cluster of risk factors which can include:
- Central obesity – excessive amounts of abdominal fat
- High triglyceride and low HDL levels
- Raised blood pressure
- Insulin resistance or glucose intolerance – body unable to use insulin efficiently
- Prothrombic state – high blood levels of fibrinogen or plasminogen activator inhibitor
- Proinflammatory state – elevated levels of C-reactive protein
The presence of some or all of these risk factors predisposes people to an increased risk of cardiovascular diseases such as coronary heart disease, peripheral vascular disease and stroke, as well as type 2 diabetes.
Estimates suggest that more than half of all type 2 diabetics have characteristics indicative of metabolic syndrome. Underlying causes of metabolic syndrome include a genetic predisposition to insulin resistance as well as obesity and physical inactivity.
Dual-acting PPAR agonists are a novel group of compounds that activate nuclear transcription factors. By activating both PPAR-alpha and PPAR-gamma receptors, they simultaneously reduce atherogenic triglycerides, raise cardioprotective HDl levels and improve insulin resistance.
They address many of the core features seen in people with metabolic syndrome and may help to reverse the underlying disease process and its adverse clinical sequelae, which includes CVD and diabetes.
DUAL PPAR AGONISTS MAY EXPAND TREATMENT OPTIONS FOR TYPE 2 DIABETICS
Drugs that target only PPAR-gamma receptors, the PPAR-gamma agonists or thiazolidinediones, are insulin-sensitisers that are already licensed for the treatment of type 2 diabetes. These agents promote fatty acid uptake and glucose metabolism so increasing sensitivity to insulin in the liver and reducing blood glucose levels. However, weight gain can occur with these agents.
Because dual-acting PPAR agonists also stimulate PPAR-alpha, which influences lipid homeostasis, they address the dyslipidaemia commonly seen in patients with type 2 diabetes as well as glucose metabolism. These therapeutic benefits should also be achieved without excessive weight gain.
GALIDA SHOWS EARLY EVIDENCE OF EFFICACY
In preliminary clinical studies Galida appeared to have a promising pharmacokinetic profile and to show dose-related effects on lipids, glucose and insulin. Clinical studies also suggested it was well tolerated.
However, safety is a critical issue with this new class of drugs as several promising candidates have already fallen by the wayside because of adverse toxicity profiles.
Adverse effects seen with some dual-acting PPAR agonists in advanced-stage development have included oedema, raised levels of hepatic enzymes and tumours in rodents. The casualties include ragaglitazar, reglitazar and MK-767, the development of which has been discontinued.
MARKETING COMMENTARY
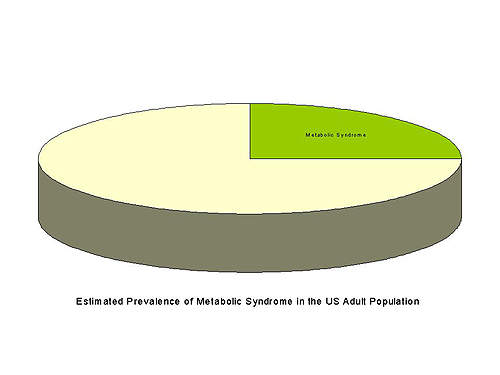
Estimated to affect around a quarter of the adult population of the USA, or around 50 million people, metabolic syndrome constitutes a major health problem. Prevalence is expected to rise further coincident with increasing rates of obesity, dyslipidaemia, insulin resistance and hypertension.
Together with lifestyle modification to reduce risk factors associated with metabolic syndrome, drug therapy is seen as a potentially important adjunct to treatment of this prevalent condition
Drugs, such as the dual-acting oral PPAR agonists, which target the cardiovascular and diabetic complications associated with metabolic syndrome, were seen as a potentially beneficial new approach to treatment. The decision to cease development of Galida is a further blow to this new therapy class.