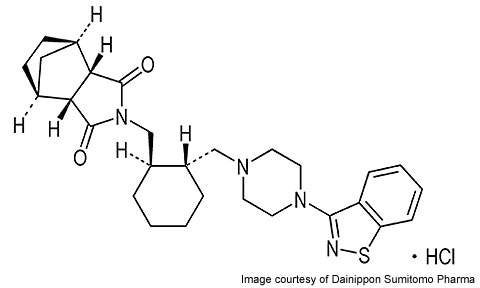
Lurasidone is an atypical antipsychotic agent developed by Japan’s Dainippon Sumitomo Pharma (DSP). The drug is indicated for the treatment of schizophrenia and is under development for the treatment of bipolar disorder.
In December 2009, DSP submitted a new drug application (NDA) for Lurasidone to the US Food and Drug Administration (FDA). The NDA was accepted for review by the FDA in March 2010. Lurasidone is expected to receive a standard ten-month review from the FDA.
Phase III trials on the drug are currently in progress. The company is planning to launch the drug in the Japanese market after these trials.
Schizophrenia
Schizophrenia is a chronic brain illness affecting both males and females across the world. It is estimated that about 1% of people in the US suffer from the disorder. Schizophrenia is caused by many factors such as the malfunctioning of a key gene in the body. The disease may also be caused by contact with certain viruses, poor nutrition before birth and other psychosocial factors. Schizophrenia is also known to be hereditary.
The disease is indicated by three symptoms – positive, negative and cognitive. Positive symptoms include abnormal behaviour, which can range from mild to severe episodes of hallucinations, delusions and movement disorders. Negative symptoms include the inability to perform daily activities, and lack of emotion and energy. The cognitive symptoms lead to problems with memory and the ability to make decisions.
Currently, antipsychotic medications that target psychotic symptoms and hallucinations are being used for treating schizophrenia. Various psychosocial treatments such as cognitive behavioural therapy, which helps in improving thinking and behaviour are also used.
Lurasidone – atypical antipsychotic agent
Lurasidone has a unique receptor-binding capability with high affinity for dopamine-2, serotonin-2A, serotonin-7, serotonin-1A and noradrenaline-2c receptors. These receptors are known to improve cognitive capabilities upon effective regulation. The drug has limited affinity for histamine-1 and acetylcholine-M1 receptors. Lurasidone is also a partial agonist for the serotonin 5HT1A receptor. It enhances the functions of muscarinic acetylcholine receptors, which are known to aid in memory and learning functions.
In clinical trials, Lurasidone has shown its efficacy in treating both positive and negative symptoms of schizophrenia. Significant improvements in the cognitive symptoms have also been noticed with the use of the drug. The safety profile of the drug is also good with minimal weight gain and metabolic effects. As a result, use of Lurasidone may not necessitate co-administration of anti-cholinergics, which are known to impair cognition.
Lurasidone clinical trials
The development of Lurasidone involved more than 40 clinical trials. Phase II trials included two six-week randomised double-blind placebo-controlled studies carried out across the US. The studies indicated that Lurasidone was well tolerated in patients and showed greater efficacy compared to the placebo group.
Phase III studies of Lurasidone included two Program to Evaluate the Antipsychotic Response to Lurasidone (PEARL) trials conducted across the world. PEARL 1 was a six-week placebo-controlled double-blind, trial which started in October 2007. The study recruited 500 patients in 51 centres across the US, Europe and Asia. The majority of the patients recruited were male with an average age of 39 years.
In May 2009, DSP announced positive results from PEARL 1 study. The results indicated that Lurasidone was more effective in treating acute schizophrenia compared with the placebo.
PEARL 2 was a randomised, fixed-dose, placebo-controlled, double-blind trial, which started in January 2008. The study was conducted in 52 centres across the US, Europe, Asia and South America. A total of 286 patients with an average age of 37.7 years were recruited for the study. Positive results from the study were announced by DSP in August 2009.
In October 2008, DSP started a third Phase III study, PEARL 3, to gather ample safety and efficacy data on Lurasidone.
DSP is also evaluating Lurasidone for bipolar disease, for which the company started two Phase III studies named PREVAIL in April 2009. Additional Phase III trials are being carried out in Japan and South Korea to enable its early development and launch in Japan.
Marketing commentary
Compared to antipsychotics currently available in the market, Lurasidone has demonstrated its ability to enhance cognitive functions. In addition, the drug’s improved safety profile is expected to make it a key player in the antipsychotics market. If approved, DSP is planning to launch Lurasidone initially in the US and later in Europe and Canada.