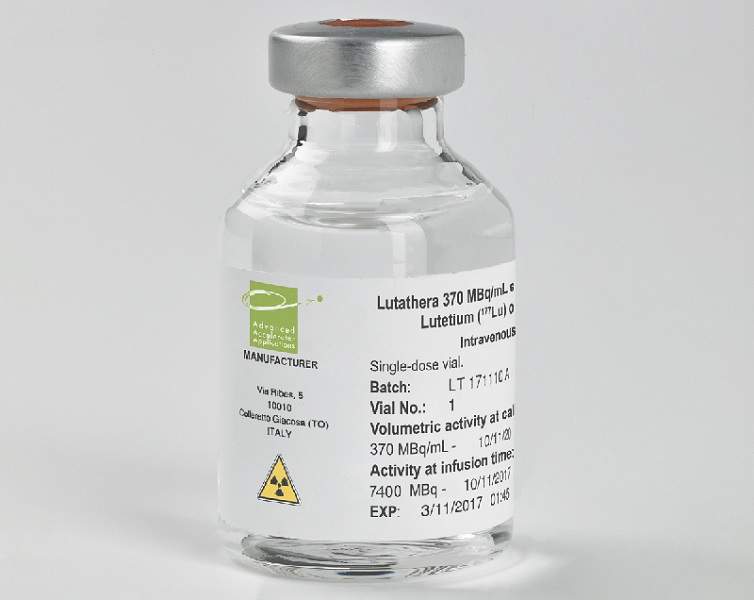
Lutathera® (lutetium Lu 177 dotatate) is a peptide receptor radionuclide therapy (PRRT) indicated for the treatment of somatostatin receptor-positive gastroenteropancreatic neuroendocrine tumours.
The drug was discovered and developed by Advanced Accelerator Applications, a Novartis company.
Lutathera was approved by the European Commission (EC) in September 2017 and by the US Food and Drug Administration (FDA) in January 2018. It was also granted orphan drug designation by the FDA and European Medicines Agency (EMA).
Gastrointestinal neuroendocrine tumours causes and symptoms
Neuroendocrine tumours (NET) are positive tumours that originate in the neuroendocrine cells of organs, including in the gastrointestinal tract, the pancreas and the lungs.
Gastrointestinal NET can occur in any part of the gastrointestinal tract, including the small intestine, large intestine and stomach.
Signs and symptoms of the disease include discomfort or pain in the abdomen or rectum, nausea, vomiting, diarrhoea, blood in the stool, anaemia, stomach ulcers, weight loss and blockage in the intestine.
According to the National Cancer Institute’s Surveillance, Epidemiology and End Results (SEER) database, the prevalence of NETs in the US was 171,321.1 in 2014. The rate of new cases reported in the US annually is approximately 6.98 per 100,000.
Lutathera’s mechanism of action
Lutathera contains lutetium Lu-177-labelled somatostatin analogue peptide. The drug belongs to the peptide receptor radionuclide therapy (PRRT) class, which carries a radioactive component for treatment.
The drug binds to the somatostatin receptor-expressing cells and destroys the tumour cells.
Lutathera is available in 370MBq / ml (10mCi / ml) single-dose vial, administered through infusion drip into the bloodstream.
Clinical trials on Lutathera
The FDA’s approval for Lutathera was based on results obtained from a randomised pivotal Phase III clinical trial named NETTER-1. This open-label, single-arm, international clinical study enrolled more than 229 patients with metastatic midgut NETs. It compared the safety and efficacy of Lutathera plus Octreotide LAR 30mg versus Octreotide LAR 60mg.
The patients were administered with Lutathera 7.4GBq plus Octreotide LAR 30mg four times for every eight weeks and Octreotide LAR 60mg for every four weeks. The primary endpoint of the study was progression-free survival (PFS) or reduction in risk of disease progression or death.
The secondary endpoints included objective response rate (ORR), overall survival and tolerability.
Results from the study demonstrated that the patients treated in the Lutathera arm met the primary endpoint and showed a 79% reduction in risk of disease progression or death compared to Octreotide LAR arm.
In addition, median PFS was 8.4 months in the Octreotide LAR arm compared to the Lutathera arm, where PFS had not been reached. A pre-planned interim overall survival analysis revealed that the patients treated with Lutathera achieved a 48% reduction in the estimated risk of death compared to Octreotide LAR arm.
Including complete and partial responses, the objective response rate was 13% for the Lutathera arm compared to 4% in the Octreotide LAR arm.
The most common grade three and four adverse reactions found in Lutathera arm were lymphopenia, increased gamma-glutamyltransferase, vomiting, nausea and elevated aspartate aminotransferase, as well as increased alanine aminotransferase, hyperglycaemia and hypokalemia.