Nesina (alogliptin) is a dipeptidyl peptidase-4 (DPP-4) inhibitor developed and marketed by Takeda Pharmaceuticals for the treatment of diabetes 2 mellitus.
The US Food and Drug Administration (FDA) approved Nesina for the treatment of diabetes 2 mellitus in January 2013. Nesina is also approved as fixed dose combination with two other drugs: Oseni (alogliptin and pioglitazone) and Kazano (alogliptin and metformin HCl).
Nesina was approved and launched in Japan in 2010. The drug is also being reviewed by European Medicines Agency (EMA)
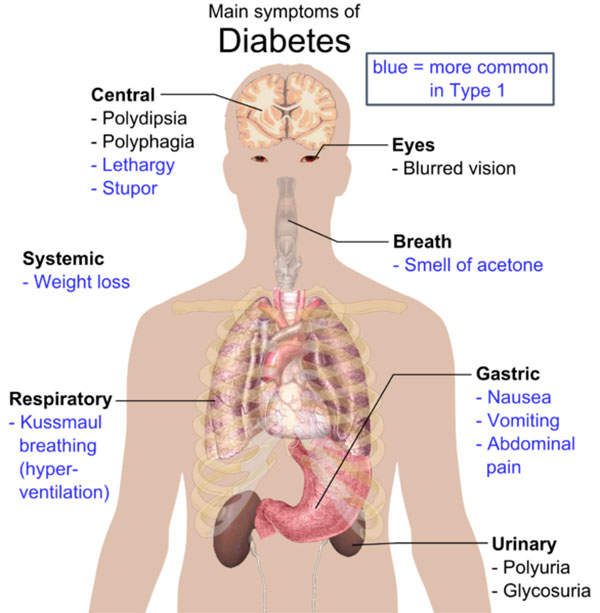
Type 2 diabetes mellitus symptoms and causes
Type 2 diabetes mellitus is a metabolic disorder that is a non-insulin dependent form of diabetes. It is characterised by high levels of sugar in the blood. The symptoms of the disease include frequent urination, constant appetite and excess thirst. It is the most common form of diabetes, which is found in about 90% to 95% of the adults diagnosed with diabetes.
The disease affects more than 23 million people in the US. The international health care costs associated with diabetes in 2012 were about $471.6bn, according to the International Diabetes Federation’s estimates. It is also estimated that the healthcare costs for the control of diabetes will go beyond $595bn by 2030.
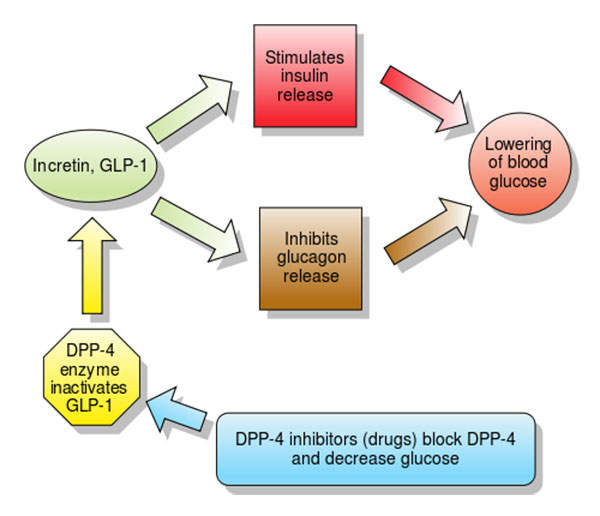
Nesina’s (alogliptin) mechanism of action
Nesina contains dipeptidyl peptidase (DPP) IV inhibitor, which reduces the glucagon and blood glucose levels. The drug increases insulin secretions and incretin levels, and also decreases gastric emptying as well as blood glucose levels.
The drug is available in a 25mg dose for oral administration.
Clinical trials of alogliptin / Nesina
The FDA approval for Nesina was based on three Phase III clinical trials that evaluated the efficacy and safety of the drug in 1,768 patients. All the three studies were conducted for four weeks as single-blind, placebo run-in period treatment, which was later followed by 26-week randomised studies.
The first Phase III clinical trial was conducted between February 2006 and July 2007. The study enrolled 329 patients with type 2 diabetes. It was a randomised, multicentre, double-blind, placebo-controlled study. The patients were randomised and administered with Nesina 12.5mg and 25mg doses or placebo once in a day. The primary endpoint of the study was to find change in glycosylated hemoglobin (A1C) from the baseline after 26 weeks. The secondary outcome measures included finding change in glycosylated hemoglobin, and fasting plasma glucose (FPG) in one to 26-week study.
The results of the study showed that the patients administered with Nesina 25mg were statistically improved in A1C and FPG from the baseline when compared to placebo. At week 26 the A1C achieved in Nesina arm was 44% compared to 23% in placebo arm. The FPG achieved in Nesina arm was about 16% from the baseline, compared 11% in the placebo arm.
The second Phase III clinical study on Nesina was conducted between February 2006 and February 2008. The study enrolled over 655 patients. It was a randomised, double blind, multicentre and parallel assignment. The patients were administered with Nesina 25mg alone, pioglitazone 30mg alone, co-administration of Nesina 12.5mg with pioglitazone 30mg or co-administration of Nesina25 mg with pioglitazone 30mg once-daily.
The study results demonstrated that the patients treated with Nesina 25mg and pioglitazone 30mg in combination improved significantly in A1C and FPG compared to Nesina 25mg alone and to pioglitazone 30mg alone in 26 weeks.
The third Phase III clinical study on Nesina was conducted between December 2009 and June 2011. It was a randomised, multicentre, double-blind and placebo controlled and parallel assignment study that enrolled about 784 patients. The patients were administered with Nesina 12.5mg twice daily, or Nesina 25mg daily, HCl 500mg or metformin HCl 1,000mg twice daily, Nesina 12.5mg in combination with metformin HCl 500mg, metformin HCl 1,000mg twice-daily, or placebo.
The results of the study showed that the patients in the Nesina 12.5mg and metformin HCl 500mg, and Nesina 12.5mg and metformin HCl 1,000mg arms statistically improved in A1C and FPG, when compared to the respective individual Nesina and metformin component regimens.
Marketing Nesina in Japan and the US
Related project
Forxiga (dapagliflozin) for Treatment of Type 2 Diabetes
Forxiga (dapagliflozin) is a type 2 diabetes drug jointly developed by AstraZeneca and Bristol-Myers Squibb (BMS).
Takeda has given the marketing rights and royalties on Nesina to Furiex Pharmaceuticals for sales based milestone in Japan, US, and other countries.
Other medications approved for type-II diabetes mellitus include Forxiga, developed by AstraZeneca and Bristol-Myers Squibb, and Byetta developed by Amylin Pharmaceuticals and Eli Lilly.