Ozempic® (semaglutide) is a glucagon-like peptide (GLP-1) receptor agonist indicated for the treatment of Type 2 diabetes in adults.
The drug was discovered and developed by Novo Nordisk, which submitted a new drug application (NDA) for the drug to the US Food and Drug Administration (FDA) under the Prescription Drug User Fee Act V (PDUFA V) regulation in December 2016.
The company received unanimous support from the FDA Endocrinologic and Metabolic Drugs Advisory Committee (EMDAC) for Ozempic’s approval in October 2017 and was approved by the FDA in December 2017 as an adjunct to diet and exercise to improve glycaemic control in adult type 2 diabetics.
Novo Nordisk also received a positive opinion from the Committee for Medicinal Products for Human Use (CHMP) under the European Medicines Agency (EMA) for the marketing authorisation application (MAA) of Ozempic® in December 2017.
The MAA was approved by the European Commission (EC) in February 2018. The drug was also approved in Canada in January 2018.
Ozempic® is currently under review by the Japanese Pharmaceuticals and Medical Devices Agency.
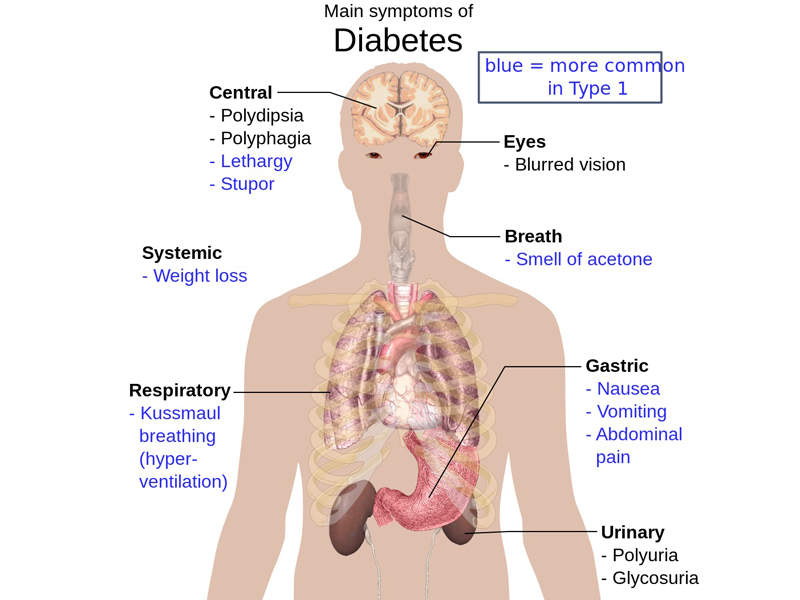
Type 2 diabetes causes and symptoms
Type 2 diabetes is a metabolic disorder that is characterised by high levels of sugar in the blood. The disease increases the risks of developing cardiovascular problems and people with the disease are four times more likely to have cardiovascular disease than those without diabetes.
The symptoms of Type 2 diabetes include frequent urination, increased appetite and excess thirst. The symptoms are found in between 85% and 95% of adult patients diagnosed with diabetes.
An estimated 28 million people in the US are affected by Type 2 diabetes.
Ozempic’s mechanism of action
Ozempic® contains an analogue of native human glucagon-like peptide-1 (GLP-1). It stimulates insulin production and lowers glucagon secretion in a glucose-dependent manner.
The drug is available in a pre-filled pen of 0.5mg and 1mg doses.
Clinical trials on Ozempic
The FDA’s approval for Ozempic® was based on results obtained from a global Phase IIIa clinical trial programme named SUSTAIN, which consisted of eight clinical trials enrolling more than 8,000 adult patients with Type 2 diabetes.
The clinical trial programme also included a two-year cardiovascular outcomes trial, which evaluated the safety of Ozempic® in adults with Type 2 diabetics that were at high risk of cardiovascular illnesses.
The study results demonstrated that patients treated with Ozempic® achieved the primary endpoint of clinically and statistically significant reductions in A1c compared to placebo, sitagliptin, extended-release formulation of exenatide and insulin glargine U100. The secondary endpoint was a reduction in body weight.
The most common adverse reactions reported were nausea, vomiting, diarrhoea, abdominal pain and constipation.
Marketing commentary on Novo Nordisk
Novo Nordisk is a global healthcare company based in Plainsboro, New Jersey, US. The company is involved in the innovation and development of treatments for chronic conditions, including diabetes, haemophilia, growth disorders and obesity. It has a workforce of more than 5,000 people in the US.
Novo Nordisk launched Ozempic® in the US market in February 2018, while European market launch is expected in the second half of 2018.
Other medicines approved and available in the market for the treatment of Type 2 diabetes include Adlyxin (lixisenatide) developed by Sanofi and Invokamet XR produced by Janssen Pharmaceuticals.