Pandemrix is an adjuvanted vaccine used in the prevention of the H1N1 (swine flu) virus infection and has been developed by GlaxoSmithKline Biologicals (GSK). The vaccine is a combination of H1N1 virus antigens and the adjuvant system AS03. The drug works by enhancing the natural immunity of the body.
Phase I clinical trials of Pandemrix were completed and the results were submitted to the Committee for Medicinal Products for Human Use (CHMP) on 14 September 2009. On 25 September 2009, the CHMP and US Food and Drug Administration issued a positive opinion and recommended the drug’s approval to the European Commission. The European Commission granted marketing authorisation for Pandemrix to GSK on 30 September 2009.
The World Health Organization (WHO) officially declared on 11 June 2009 that Pandemrix should be used only for the Influenza A (H1N1) flu pandemic.
On 15 May 2009, GSK received orders from the US, France and Finland for the supply of the vaccine and agreed to supply the UK Government with 60 million doses of vaccine, the French Government with 50 million doses of vaccine and Finland with 5.3 million doses of vaccine.
The total number of doses of Pandemrix ordered reached 291 million by July 2009. Out of these, 195 million doses of the vaccine worth $250m have to be supplied to the US Government as per the contract signed by GSK. Later, nine other countries ordered a further 96 million doses of Pandemrix.
It was expected that the first doses would be supplied to governments from September onwards and shipments between late 2009 and early 2010.
On 14 September 2009, GSK released the results of its first clinical trial, stating the use of its vaccine. By September 2009, the results of clinical trials on more than 39,000 people demonstrated that AS03 is safe when used with the influenza vaccine.
The authorisation of Pandemrix was allowed under ‘Exceptional Circumstances’, as the authorisation had to be awarded based only on Phase I clinical trials.
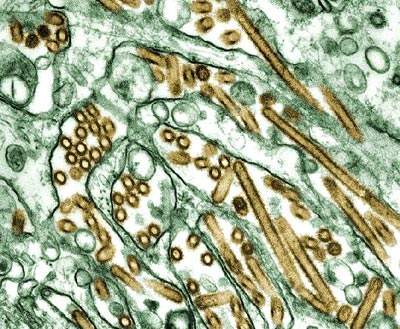
Swine flu (H1N1)
The swine flu (H1N1) virus first came to the attention of doctors when an outbreak started in Mexico during April 2009, then creating a news frenzy as the number of cases increased at an alarming rate.
The symptoms of the disease include high fever, cough, runny or stuffy nose, sore throat, body aches, chills, fatigue or tiredness, extreme diarrhoea and vomiting.
In April 2009, when it was confirmed that the flu can transmit from human to human, the WHO raised the worldwide flu alert level from four to five, a level away from full pandemic. On 12 June 2009, the level was increased to six because the virus had shown higher spread within a population for a sustained period.
By 8 July 2009, the WHO had reported a total of 94,500 cases of influenza A (H1N1) in humans in 122 countries.
Targeting swine flu
Pandemrix contains parts of the flu strain A/California/7/2009 (H1N1) v-like strain (X-179A) and also contains GSK’s proprietary adjuvant system AS03. The AS03 adjuvant contains tocopherol (vitamin E). The clinical trials of the H5N1 influenza strain proved that the adjuvant provides protection even if the strain drifts. Pandemrix contains small amounts of haemagglutinins (proteins from the surface) of a virus called A(H1N1)v and allows the immune system to defend itself against a disease.
The vaccine is administered by injection into the shoulder muscle, preferably as two single doses at least three weeks apart. A single dose of the vaccine consists of 3.75ug of antigen and AS03.
Pandemrix should not be administered to people who have had an anaphylactic reaction to any of the components of the vaccine. The vaccine should be given only with a prescription.
The common side effects identified with the administration of Pandemrix are headache, joint and muscle pains, reactions at the site of the injection, fever and fatigue.
Clinical trials demonstrate efficacy of Pandemrix
A total of 16 clinical trials were designed and out of these the Phase I trials were completed. The Phase I trials were designed to check the tolerability and immunogenicity of the pandemic (H1N1) adjuvanted vaccine (containing 5.25ug of antigen) in comparison with an unadjuvanted (containing 21ug) test study formulation. These trials were conducted in Germany by GSK and involved 130 healthy volunteers between 18 and 60 years of age. All clinical trials have been designed to follow the subjects for up to a year to notice the safety of the vaccine.
The European Medicines Agency (EMEA) announced that there was less clinical experience available on adults to whom a higher amount of Pandemrix antigen was given. It also stated that there is no clinical experience in older people, children and pregnant women.
After the EMEA’s announcement, GSK started conducting additional 15 studies in over 9,000 subjects. They included healthy adults in Europe, Canada and the US. The drug maker also announced that it will run initial trials in a healthy population but not in pregnant women.
Even though there is no clinical data for pregnant women and children, these groups are given major importance by the European Commission and WHO for the administration of the vaccine.
Results of the first clinical trials released by GSK in September 2009 stated that one dose of Pandemrix can generate a strong immune response that is higher than the immunogenicity criteria stated by international licensing authorities.
At first, Pandemrix was developed as a ‘mock-up’ vaccine, by selecting an H5N1 strain called A/Vietnam/1194/2004. The company noticed the ability of this mock-up vaccine as it increased the production of antibodies.
The mock-up vaccine was proven to produce the antibodies in a minimum of 70% of people on whom studies were carried out. This demonstrated that the vaccine provides an appropriate level of protection.
Based on the data provided by mock-up vaccine and strain drifts, the CHMP announced that Pandemrix has more benefits than risks for the prevention of swine flu.
Marketing commentary
When the Pandemrix vaccine first became available, it was expected that the demand would be greater than the supply. It is expected that the gap may reduce as more vaccine is released over time.
GSK is conducting meetings with more than 50 governments of developed and developing countries to determine supplies of Pandemrix. Meanwhile, governments are already evaluating and determining their vaccination policies and target groups within their specific countries.
GSK has allotted 20% of the Pandemrix production to its manufacturing facility in Canada, to distribute the vaccine to developing countries from September 2009.
GSK is following the policy of tiered pricing for Pandemrix, based on the World Bank classification of countries and GAVI eligibility.