Prexige (lumiracoxib) is an innovative COX-2 inhibitor developed by Novartis for the relief of pain and inflammation associated with musculoskeletal disorders and other causes of acute and chronic nociceptive pain.
In November 2002, Novartis filed for regulatory approval of Prexige in both the US and Europe, hoping to secure approval in 2003 for a global launch in 2004. After a series of setbacks, Prexige finally secured EMEA approval towards the end of 2006 and was launched first in the UK, the reference state in the EU’s mutual recognition procedure. However, the drug failed to secure FDA approval; Novartis received a ‘not approvable’ letter from the FDA in October 2007.
The FDA ruling followed shortly after the company’s decision to withdraw the drug at all doses from the Australian market.
In Australia, one of the first countries to approve Prexige, several patients had experienced serious hepatic side effects, including two deaths from serious liver injury. Further cases of hepatic toxicity have since emerged and the drug has now been withdrawn from the Canadian market and, most recently, the UK.
COX-2 inhibitors represent an advance in NSAID research
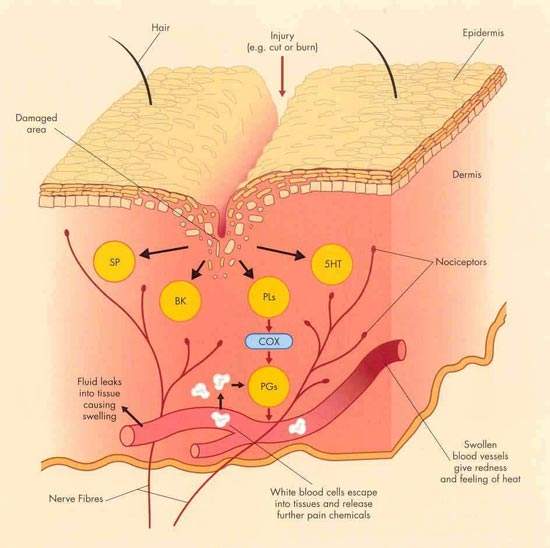
Traditional non-steroidal anti-inflammatory drugs (NSAIDs) such as ibuprofen and naproxen have been the mainstay of treatment of mild to moderate pain and inflammation arising from headache, toothache, joint pain and period pain, to name a few.
Their analgesic, anti-inflammatory and antipyretic action is due to inhibition of cyclo-oxygenase (COX) enzymes in damaged tissue. However, because they inhibit both COX-1 and COX-2 isoenzymes they are associated with adverse gastrointestinal side effects such as stomach irritation, gastroduodenal ulcers and bleeding. These side effects can seriously limit their clinical usefulness.
The development of the selective COX-2 inhibitors, which primarily target the COX-2 isoenzyme associated with tissue injury, was seen as a major advance. These agents retain the analgesic and anti-inflammatory properties of the traditional NSAIDs but cause less gastrointestinal toxicity because they do not inhibit the gastro-protective effects of COX-1.
Prexige shows efficacy and gastrointestinal safety in clinical trials
The efficacy and safety of Prexige has been investigated in a series of clinical trials. Overall, the studies indicate that Prexige is as effective as existing agents in reducing pain and inflammation but has a superior gastrointestinal safety profile compared with traditional NSAIDs.
In the treatment of patients with osteoarthritis, Prexige exhibited efficacy equal to that of diclofenac, considered the ‘gold standard’ in Europe. An analysis of responder rates among 583 patients with osteoarthritis showed that Prexige 400mg/day provided the same degree of efficacy as high-dose diclofenac (75mg twice daily). Treatment response was defined as a 20% reduction in osteoarthritis pain intensity.
With respect to gastrointestinal safety, studies show that the incidence of gastrointestinal side effects with Prexige is similar to that of the COX-2 inhibitor celecoxib but significantly lower than commonly used NSAIDs such as ibuprofen and naproxen. In one placebo-controlled study for example, 65% of patients on naproxen (500mg twice daily) experienced gastrointestinal erosions. In contrast, there were no erosions in the Prexige or placebo groups.
Target trial data released
The advent of the selective COX-2 inhibitors has undoubtedly advanced the treatment of pain and inflammation. However, these agents can cause some gastrointestinal irritation and concerns have been raised about potential cardiotoxicity.
To address these safety issues, Novartis initiated the 12-month TARGET (Therapeutic Arthritis Research & Gastrointestinal Event Trial) in 18,000 male and female patients with symptomatic osteoarthritis.
TARGET is the first COX-2 inhibitor gastrointestinal safety trial where cardiovascular safety will be assessed as one of the pre-specified endpoints.
Results from the TARGET trial, which were published in August 2004, showed that Prexige was associated with a significantly lower incidence of gastrointestinal ulcers compared with comparator drugs (the non-specific NSAIDs ibuprofen and naproxen) but, importantly, was not associated with any increased cardiovascular risk.
Marketing commentary
Drugs for relief of pain and inflammation constitute a large segment of the CNS market, in which COX-2 inhibitors were a growing sector before the withdrawal of rofecoxib (Vioxx) in 2004. Concern about the cardiovascular safety of this new class of medicines has certainly dampened enthusiasm. Hepatic safety may now be an issue following the decision to withdraw Prexige from all major markets.
COX-2 inhibitors had been widely used in the treatment of musculoskeletal disorders such as osteoarthritis, the most common form of arthritis. Over 25 million Europeans and more than 20 million people in the US are affected by osteoarthritis, most of whom require long-term pain relief. Thus, there is a large market for new and improved pain-relieving medicines.