AstraZeneca’s Tagrisso maintains momentum in NSCLC with FLAURA2 results
Tagrisso’s advantage over its competitors in the US is emphasised by its category 1 recommendation by the NCCN.
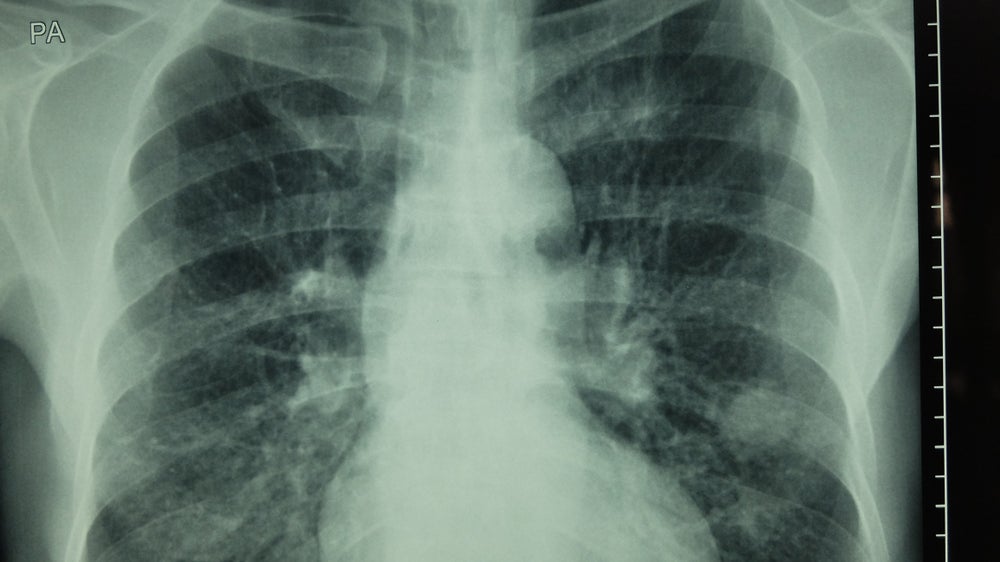
Tagrisso’s advantage over its competitors in the US is emphasised by its category 1 recommendation by the NCCN.



The observational study will assess the clinical and workflow benefits of using Hyperfine’s Swoop system in detecting amyloid abnormalities in Alzheimer’s patients.
The pharmaceutical industry continues to be a hotbed of patent innovation. Activity is driven by the evolution of new treatment...
Acarix starts clinical workflow study for its AI-powered cardiac diagnostic
Cleerly touts new data for AI cardiovascular software
Moderna’s Phase III Covid-19 vaccine trial meets primary endpoints
Gedeon Richter adopts TransPerfect’s trial management solutions