Spritam (levetiracetam) is an adjunctive therapy indicated for the treatment of seizures in patients with epilepsy, the fourth most common neurological disorder affecting people of all age groups.
The drug was discovered and developed by Aprecia Pharmaceuticals using its proprietary ZipDose technology platform that uses three-dimensional printing (3DP) to create fast-melting material.
3DP technology is commonly used to manufacture medical devices, but was never used for drug manufacturing.
In August 2015, Spritam became the first 3D-printed drug approved by US Food and Drug Administration (FDA) as a prescription adjunctive therapy for treating myoclonic seizures, partial onset seizures and primary generalised tonic-clonic seizures in patients with epilepsy.
Aprecia submitted the new drug application (NDA) for the drug to the FDA December 2014. The drug was launched in the US market in March 2016.
Epilepsy and its effects
Epilepsy is a chronic neurological disorder that affects brain function, leading to recurrent and unprovoked seizures, which cause abnormal bursts in the neurological system.
People with epilepsy may experience more than one type of seizure, with varying severity, along with other neurological problems.
More than three million people, including 460,000 children, are estimated to have been diagnosed with epilepsy in the US.
Spritam’s mechanism of action, dosage and administration
Spritam contains an active ingredient called levetiracetam, whose mechanism of action in exerting an anti-epileptic effect is not clearly known.
The drug is available in the form of a tablet in various doses, including 250mg, 500mg, 750mg and 1,000mg. It is to be administered orally and disintegrates in a mean time of 11 seconds with a sip of liquid.
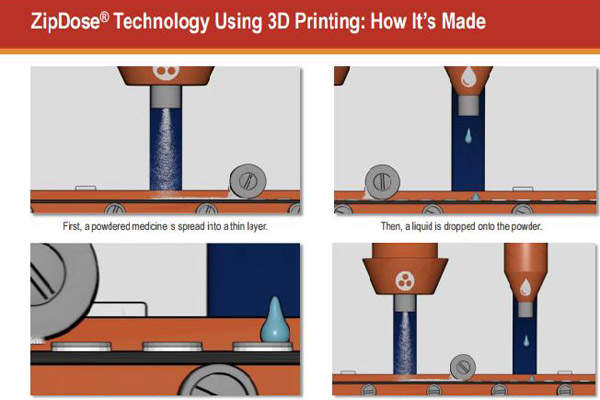
ZipDose technology and 3D printing
Zonegran (Zonisamide) is an anti-epileptic drug indicated for the treatment of partial seizures in adults with epilepsy with or without secondary generalisation.
ZipDose technology creates a porous formulation using a 3DP process that binds powders without compression. The method enables delivery of high drug strengths of up to 1,000mg in a single dose.
Drugs formulated using ZipDose technology are specially designed for people with swallowing difficulties and those who skip regular drug doses, resulting in ineffective treatment outcomes.
Poor adherence to the drug routine especially in epileptics may lead to serious complications, including frequent seizures.
According to a survey conducted on epilepsy patients, 71% reported they often forget / miss / skip their dose on time, while half of them reported having a seizure after a missed dose.
Spritam, formulated with 3DP technology, is considered a breakthrough in the treatment of epilepsy. The technology enables immediate disintegration of the drug with a sip of water, making it easy for the patients to take the drug, even in high doses.
Clinical trials on Spritam
The FDA approval for the drug was based on three multi-centre, double-blind, randomised, placebo-controlled clinical studies conducted on patients with refractory partial onset seizures with or without secondary generalisation.
A total of 904 patients were enrolled in the studies, randomised to either a placebo or Spritam 1,000mg, 2,000mg, or 3,000mg a day.
Study one and study two enrolled patients with refractory partial onset seizures for at least two years and had taken two or more classical anti-epileptic drugs (AED).
The third study enrolled patients with refractory partial onset seizures for at least one year and had taken one classical AED. Subjects had an experience of at least two partial onset seizures during the four-week period prior to selection.
Study one was a double-blind, parallel-group, placebo-controlled study conducted at 41 sites in the US. It compared Spritam 1,000mg/day, 3,000mg/day, and a placebo given in equal doses twice a day.
The study was conducted for 18 weeks, of which the first six weeks were for a titration period followed by a 12-week fixed dose evaluation period.
Study two was a double-blind, placebo-controlled, crossover study conducted at 62 sites in Europe. It compared Spirtam 1,000mg/day, Spritam 2,000mg/day and a placebo administered in equal doses twice a day.
The third study was a double-blind, parallel-group, placebo-controlled study conducted at 47 centres in Europe and compared Spritam 3,000mg/day with a placebo.
Subjects were given the drug in two divided doses. The 16-week treatment comprised a four-week titration period, followed by a 12-week fixed-dose evaluation period.
The primary outcome for all studies was the percentage reduction in partial seizure frequency a week compared to a placebo, over the treatment period. Secondary outcome variables included the responder rate.
Study one demonstrated statistically significant results with 37% subjects from the Spritam 1,000mg/day group and 39.6% from the Spritam 3,000mg/day group, achieving more than 50% reduction in weekly seizure rates from baseline.
Study two also demonstrated statistically significant results with 20.8% subjects from the Spritam 1,000mg/day group and 35.2% from the Spritam 2,000mg/day group, achieving more than 50% reduction in weekly seizure rates from baseline.
The results of the third study was statistically significant, with 44.6% subjects who received Spritam, compared to 19.6% of subjects who received a placebo, achieved more than 50% reduction in weekly seizure rates from baseline.
The most common adverse reactions recorded during the study were sleepiness, weakness, dizziness and infection. The most common side effects in children were tiredness, acting aggressive, nasal congestion, decreased appetite and irritability.