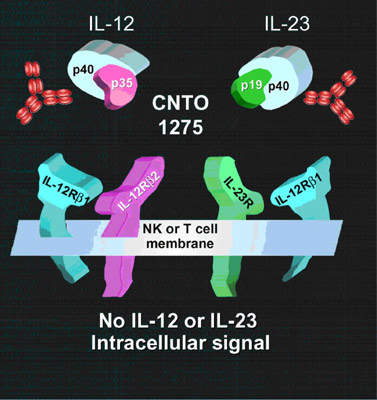
Ustekinumab (CNTO 1275), a human anti-interleukin 12 (IL-12) and anti-interleukin 23 (IL-23) monoclonal antibody (MAb), is a novel investigational therapy for plaque psoriasis developed by Centocor (a subsidiary of J&J).
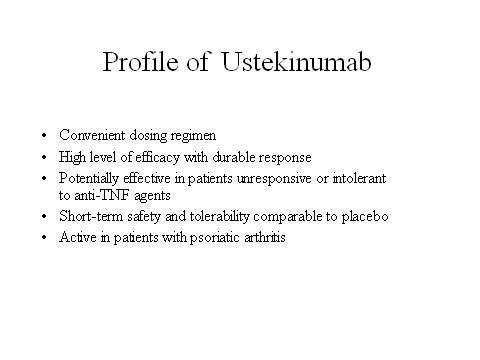
Ustekinumab is emerging as an exciting new biological therapy for moderate-to-severe plaque psoriasis, a condition for which there is still significant unmet clinical need. The drug has undergone Phase II and III clinical trials, which gave positive results.
In early December 2007, Centocor filed for regulatory approval of ustekinumab with the US Food and Drug Administration (FDA) and J&J’s European subsidiary Janssen-Cilag with the European Medicines Agency (EMEA). In June 2008, the FDA’s Dermatologic and Ophthalmic Drugs Advisory Committee recommended the approval of ustekinumab.
The FDA approved the drug on 19 September 2009 for treating adult patients who have prior exposure of phototherapy or systemic therapy. The FDA has observed, however, that the drug can decrease immunity in patients, making them vulnerable to cancer and dangerous infections caused by viruses, fungi and bacteria.
The EMEA’s Committee for Medicinal Products for Human Use (CHMP) gave a positive opinion on ustekinumab in November 2008. In January 2009, the European Commission approved ustekinumab. The approval granted use of ustekinumab in 27 countries across Europe for treating moderate to severe plaque psoriasis in adults. The drug was also approved by the Canadian Health Authority in December 2008.
The burden of plaque psoriasis
Plaque psoriasis is a common skin disorder, in which patients develop characteristic raised erythematous skin lesions with silvery-white scales. Overproduction of skin cells occurs predominantly on the elbows, knees, buttocks, trunk and scalp. Up to 40% of psoriasis patients develop psoriatic arthritis, a destructive and painful joint disease.
Although not a life-threatening disease, plaque psoriasis can be deeply disfiguring. It adversely affects patient quality of life (QoL). Studies indicate that patients with plaque psoriasis experience physical and psychosocial morbidity on a par with patients suffering congestive heart failure, diabetes, cancer and depression.
The discovery that psoriasis was a chronic autoimmune disease led to the development of biological therapies designed to target aberrant cell-mediated immune mechanisms. Ustekinumab targets Il-12 and Il-23, two cytokines that are believed to play a role in the activation of T cells and hence immune-mediated inflammatory diseases. Ustekinumab has a different mode of action to currently available biological therapies for psoriasis.
Ustekinumab shows efficacy in plaque psoriasis
In the large Psoriasis Followed by Long-Term Extension (PHOENIX)-2 trial, more than two thirds of patients treated with ustekinumab experienced at least a 75% reduction in psoriasis at week 12, as measured by PASI 75 (primary endpoint). This compared with only 4% of placebo recipients.
Among patients who received a further dose of ustekinumab at week 16, a substantial proportion maintained the PASI 75 response through to week 28. Importantly, symptom improvement was accompanied by significant improvements in QoL. This included those patients who had initially been assigned placebo and then crossed over to the ustekinumab treatment arm.
In the ongoing T12 study, ustekinumab is being compared head-to-head with etanercept, an anti-TNF agent. TNF inhibitors are an important class of agents for the treatment of autoimmune diseases, including plaque psoriasis.
As with all biological therapies that modulate immune mechanisms, long-term safety will be an important issue. This will be addressed in the current PHOENIX-2 trial, which will run for at least five years. The PHOENIX-2 trial has enrolled patients who are above 18 years old and have suffered with psoriasis for at least six months.
Ustekinumab also shows efficacy in psoriatic arthritis
Psoriatic arthritis is a common complication of psoriasis. A Phase II placebo-controlled trial, in which ustekinumab was administered to patients with psoriatic arthritis, showed that ustekinumab was significantly more effective than placebo in relieving tender and swollen joints. Potentially, its use could be extended beyond plaque psoriasis to also include psoriatic arthritis.
Centocor’s Remicade, an anti-TNF MAb, is already an approved treatment for both psoriasis and psoriatic arthritis. For patients with psoriatic arthritis, it is indicated for inhibiting the progression of structural damage to joints together with improved physical functioning.
Marketing commentary
Estimates suggest that 125 million people worldwide are affected with psoriasis, of whom around 25% have moderate to severe disease. Most psoriasis patients require lifelong treatment to control symptoms such as itching (pruritis), scaling, pain and bleeding from the thickened skin lesions.
While traditional topical therapies are still widely used, the advent of the biological therapies, such as anti-TNF agents, has revolutionised the treatment of plaque psoriasis.
With a different mode of action to existing biologicals and a convenient dosing regimen (recommended dosage of the drug is 45mg and is injected for 12 weeks), ustekinumab could provide dermatologists with another valuable treatment option for this chronic skin disorder.