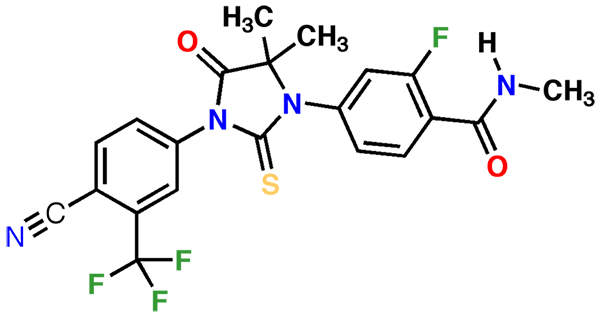
Xtandi (enzalutamide) is an androgen receptor inhibitor indicated for the treatment of metastatic castration-resistant prostate cancer (mCRPC). It is jointly developed and manufactured by Medivation and Astellas Pharma.
The new drug application (NDA) for Xtandi was accepted by the US Food and Drug Administration (FDA) in July 2012. The drug was granted priority review designation with a Prescription Drug User Fee Act (PDUFA) action date for November 2012.
However, in August 2012, the FDA approved the drug three months earlier than the PDUFA date for the treatment of mCRPC patients previously treated with docetaxel-based chemotherapy.
In June 2013, the European Commission (EC) granted marketing authorisation for Xtandi for the treatment of adult men with mCRPC, who were earlier treated with docetaxel chemotherapy.
The FDA further approved Xtandi in September 2014 as a treatment for men with mCRPC, who were not treated with chemotherapy. The company submitted supplemental new drug application (sNDA) for the drug based on the results from the Phase 3 clinical trial Prevail.
The EC also granted a variation to amend the marketing authorisation for Xtandi, which is now approved for the treatment of adult men with mCRPC for whom chemotherapy is not yet indicated. A variation application to amend the marketing authorisation was submitted to the European Medicines Agency (EMA) on 2 April 2014.
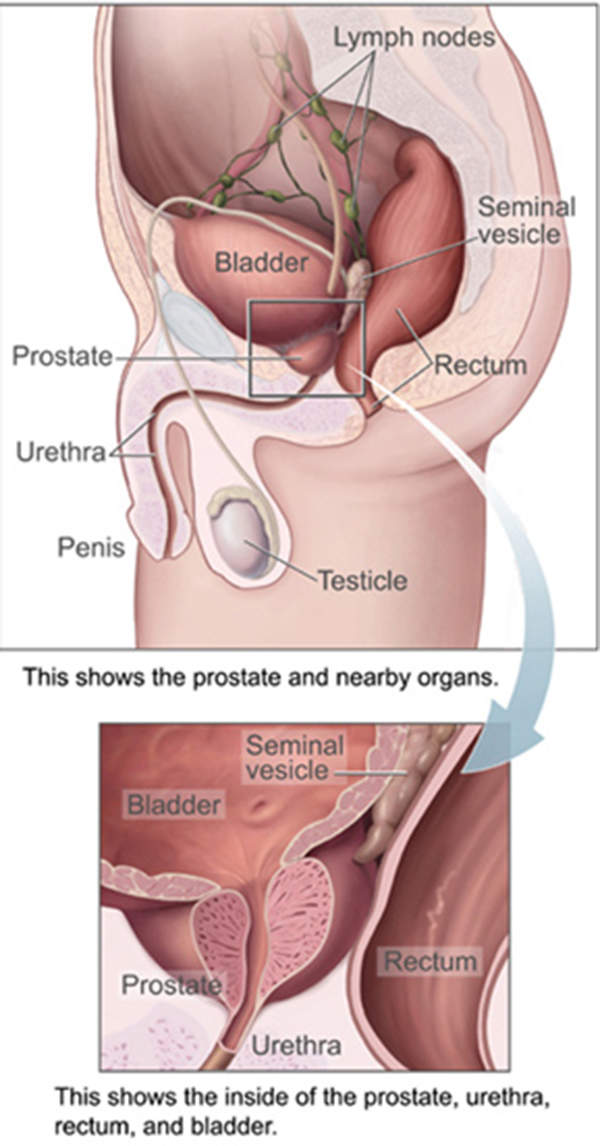
Metastatic castration-resistant prostate cancer
Prostate cancer originates in the prostate gland, which is the reproductive system of a man. The disease in metastatic or late stage spreads to other parts of the body, resulting in what is known as metastatic castration-resistant prostate cancer (mCRPC). mCRPC is the second most deadly disease in the US after lung cancer.
According to American Cancer Society estimates, a total of 241,740 new cases of the disease were diagnosed in the US in 2012, with 28,170 patients expected to die.
Xtandi mechanism of action
Xtandi contains an androgen receptor antagonist. The drug works by inhibiting androgen binding to androgen receptors, and restrains androgen receptor nuclear translocation and interaction with DNA.
The drug is administered in 40mg capsules four times a day, with a total dose of 160mg a day.
Phase III clinical trials of enzalutamide
The FDA approval for Xtandi was based on a Phase III clinical study known as AFFIRM. It was conducted between September 2009 and July 2012. The randomised, global, placebo-controlled study enrolled 1,199 patients with mCRPC. It evaluated the safety and efficacy of Xtandi. Patients who were administered with docetaxel were randomly given Xtandi 160mg oral dose or placebo once daily. The primary endpoint of the study was finding the overall survival (OS). The secondary outcome measures included finding radiographic progression-free survival, FACT-P quality of life, and time to prostate-specific antigen (PSA) progression.
The results of the study demonstrated that patients administered with Xtandi showed statistically significant improvement in median OS, when compared to the patients treated with placebo. The median OS in the Xtandi-administered group was 18.4 months, whereas it was 13.6 months in the placebo group. Approximately 37% of the patients administered with Xtandi experienced lower risk of death when compared with placebo. The number of deaths reported in the Xtandi arm was 38.5% while it was 53.1% in the placebo arm .
The most common adverse reactions found during the clinical study were back pain, asthenia, diarrhoea, headache, muscular weakness, insomnia, anxiety, upper respiratory infection, and hypertension.
An open label safety study will be conducted on Xtandi in patients who are at high risk for seizure. The study will enrol 350 subjects and its results are expected in 2019.
Marketing Xtandi in the US
Related project
Provenge – Treatment for Advanced Prostate Cancer
Provenge (sipuleucel-T) is an autologous cellular immunotherapy used intravenously in the form of infusion for the treatment of men with advanced prostate cancer. The drug was developed by the Dendreon Corporation.
Medivation and Astellas launched Xtandi in the US market in mid-September 2012. Medivation will receive $35m in milestone payments from Astellas. Medivation will employ a sales force of 60 for the promotion of the drug in the US market, while Astellas will employ 90 sales people.
The new drug will face competition from other approved medications available in the market for the same indication, such as Provenge developed by Dendreon Corporation, Sutent manufactured by Pfizer, and Abiraterone developed by BTG and Johnson & Johnson.