Zohydro (hydrocodone) is a single-entity oral drug indicated for the treatment of chronic pain. San Diego-based pharmaceutical company Zogenix developed and commercialised the drug in the US.
Zogenix submitted a new drug application (NDA) to the US Food and Drug Administration (FDA) for Zohydro extended release (ER) capsules in May 2012.
Zohydro ER was approved by the FDA in October 2013, making it the first such therapy without acetaminophen. Overdoses of acetaminophen over long periods cause liver injury.
In addition, the new formulation of Zohydro ER (hydrocodone bitartrate) capsules with BeadTekTM, a formulation technology that provides abuse-deterrent properties without altering hydrocodone’s release properties when Zohydro ER is used as intended, was approved by the US FDA in January 2015.
Chronic pain
Chronic pain is a condition that affects any part of the body and lasts for long periods of time. It is persistent and the pain signals keep firing in the nervous system for weeks, months and years. It can suppress the immune system, decrease the body’s production of natural painkillers and increase anxiety, stress, depression and anger.
It is estimated that approximately 116 million people in the US are affected by chronic pain and its economic costs throughout the nation are roughly between $560bn and $635bn a year.
One of the treatments for chronic pain is the use of opioid pain reliever hydrocodone. The drugs currently available in the market are combination products of hydrocodone with mostly acetaminophen or ibuprofen.
Zohydro – single-entity hydrocodone therapy
Zohydro is a single-entity hydrocodone therapy, which contains a semi-synthetic opioid. The detailed mechanism of action of the drug is not known. It is believed to control moderate to severe pain when administered every 12h. It does not contain any acetaminophen, which causes liver toxicity. The extended-release capsules are available in 10mg, 20mg, 30mg, 40mg and 50mg doses.
Zohydro has been developed using Spheroidal Oral Drug Absorption System (SODAS), a drug delivery technology patented by Alkermes Pharma Ireland. In 2012, Zogenix signed a licence agreement with Alkermes to pay $1m for using the patented technology.
Clinical trials
Zogenix submitted the NDA to FDA for Zohydro based on two Phase III clinical trials known as 801 and 802 studies. The studies enrolled more than 1,100 patients with chronic pain.
Related feature
Snapshot: The market for neuropathic pain therapeutics
As conditions such as diabetes become more common, the market for new pain drugs is becoming increasingly competitive.
Zogenix conducted the 801 efficacy study on Zohydro between March 2010 and October 2011. It was an efficacy study which met its primary efficacy endpoint by demonstrating that Zohydro significantly improved chronic pain relief when compared to placebo. The study also met two secondary endpoints of improvement of overall satisfaction of medication and the proportion of patients with at least 30% improvement in pain intensity.
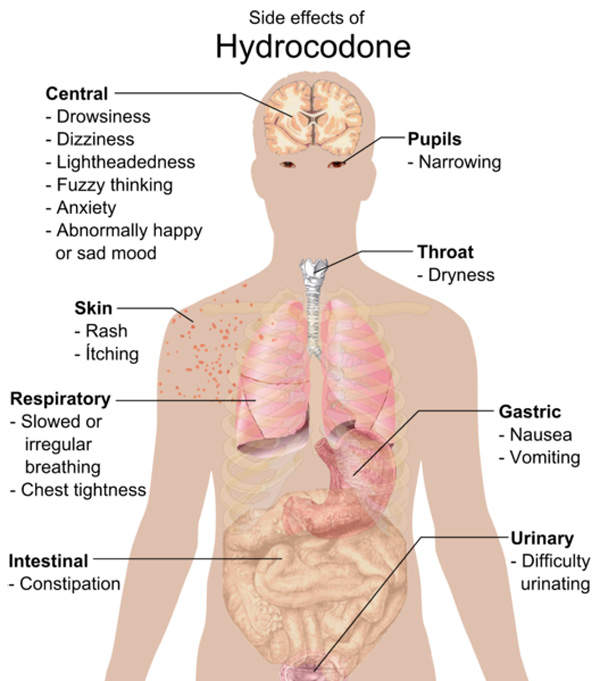
The most commonly reported side effects of Zohydro were nausea, constipation, somnolence, headache, fatigue, dizziness, vomiting, dry mouth and pruritus.
Zogenix conducted the 802 safety study on Zohydro between May 2010 and January 2012. It was an open label study. The patients were administered with Zohydro for 12 months. The study results showed that the drug was safe and well tolerated, and the incidence of adverse events was consistent with study 801.
The primary endpoint of the study was the evaluation of long-term safety and tolerability of Zohydro.
Marketing commentary
Zohydro is the first hydrocodone product to offer the benefit of less frequent dosing. It is also the first hydrocodone drug without acetaminophen combination.
Other hydrocodone drugs currently available in the market are combination drugs that may result in liver toxicity from prolonged use.
Denmark-based pharmaceutical company Egalet is also developing a pure hydrocodone product for treating chronic pain. The drug is currently in Phase I clinical trials and may hit the market by 2015.
Related projects
Cymbalta (Duloxetine HCl) for Chronic Musculoskeletal Pain Management
Cymbalta (Duloxetine hydrochloride) is indicated for treating chronic musculoskeletal pain as a result of osteoarthritis and back pain. The drug was discovered by Eli Lilly.
Sativex – Investigational Cannabis-Based Treatment for Pain and Multiple Sclerosis
Developed by GW Pharmaceuticals, Sativex is a whole plant medicinal cannabis extract indicated for the relief of multiple sclerosis (MS) symptoms and the treatment of severe neuropathic-related cancer pain.