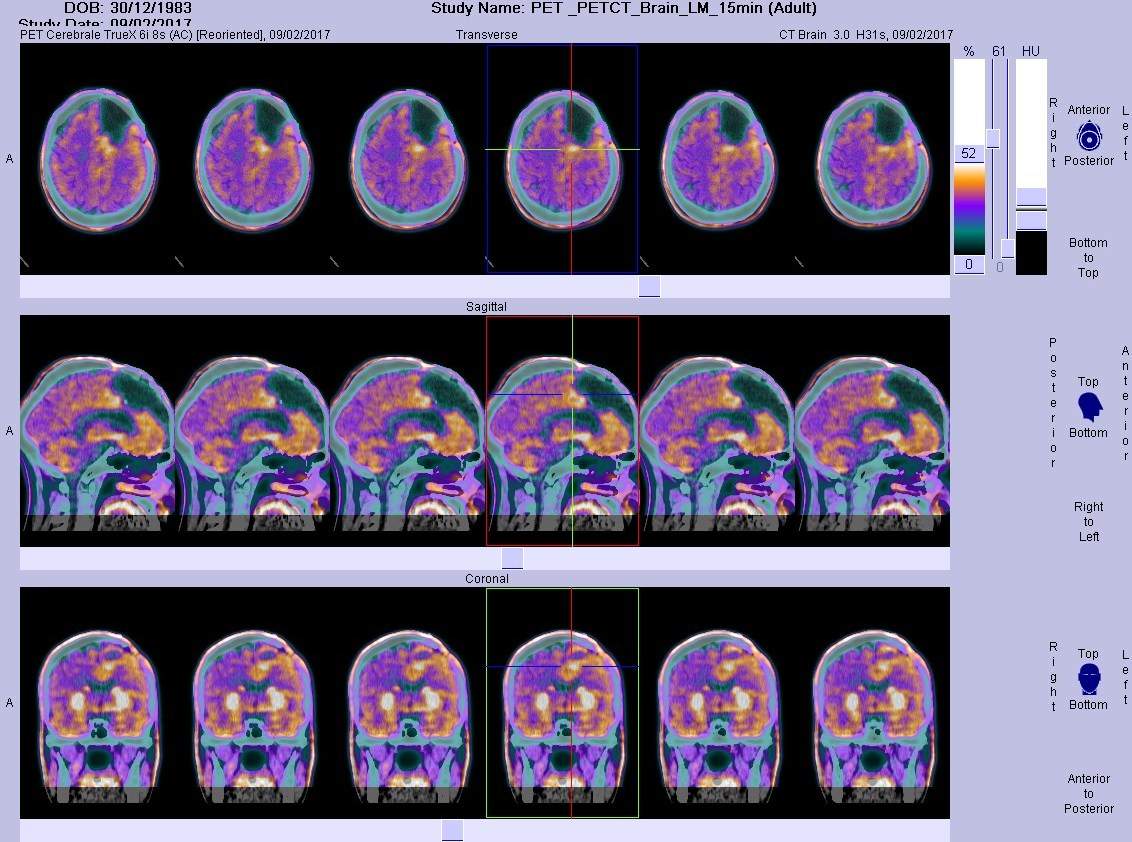
Developers have historically faced difficulties in finding effective agents to treat patients with glioblastoma multiforme (GBM), the most common form of grade IV brain cancer. The emergence of immune checkpoint inhibitors has shown promise in other tumour types, however they have not yet been successful in showing benefit for patients with GBM. Why are only a small percentage of patients with GBM showing a benefit from these PD-1 inhibitors, and what strategies could be explored to increase this percentage? The answer to this relates to the so-called ‘cold’ property of the tumours that are observed in GBM.
GBM is considered to be one of the most ‘immune-cold’ tumours relative to other cancers; this limits the effectiveness of immunotherapies. ‘Cold’ versus ‘hot’ refers to how densely the tumours are infiltrated by the immune system’s army of cells. In general, cold GBM tumours have very few T-cell infiltrates, and those within the tumour are scattered. Additionally, GBM tumours are considered to have one of the lowest rates of tumour mutation burden (TMB), a measure of the number of mutations in a tumour genome, relative to other cancers. This is believed to result in fewer cancer-specific neoantigens and poor immunogenicity of the tumour, thus leading to poor responses to immunotherapy.
In April 2017, Bristol-Myers Squibb (BMS) announced that the Phase III trial CheckMate 143, evaluating Opdivo monotherapy for patients with recurrent GBM, did not meet its primary endpoint of overall survival (OS) and this arm of the trial was terminated prematurely. Although the failure of this trial could be considered a barrier to progression in the space, it has spurred an effort to reassess research and development strategies.
The CheckMate 143 trial failure suggests that targeting newly diagnosed GBM patients with combinations of immunotherapies may offer a greater benefit. More evidence has been released that GBM tumours become even more immunosuppressive once the tumours recur, especially with first-line treatment of temozolomide. Therefore, targeting the newly diagnosed (temozolomide-naïve) GBM patient population could be a more effective strategy.
A second method to improve response rates in patients could be to increase T-cell infiltration into the tumour, for example by combining immune-checkpoint inhibitors with vaccines to activate these immune-cold tumours. If antigen-specific T-cells have been induced, this could potentially force T-cells into the tumour, leading to warmer tumours and more significant responses for GBM patients. BMS currently has two ongoing trials of Opdivo with radiotherapy and with and without temozolomide for patients with newly diagnosed GBM, named CheckMate-498 and CheckMate-548.
Ultimately, the use of immune-checkpoint inhibitors as a monotherapy is observed to be limited for patients with immune-cold GBM tumours. A two-pronged strategy, featuring combinations with other treatment modalities (radiotherapy, or with other immune-related therapies such as oncolytic viruses or DNA vaccines) coupled with targeting the newly diagnosed GBM patient population, may lead to more promising outcomes for patients with this highly aggressive form of brain cancer.
How well do you really know your competitors?
Access the most comprehensive Company Profiles on the market, powered by GlobalData. Save hours of research. Gain competitive edge.

Thank you!
Your download email will arrive shortly
Not ready to buy yet? Download a free sample
We are confident about the unique quality of our Company Profiles. However, we want you to make the most beneficial decision for your business, so we offer a free sample that you can download by submitting the below form
By GlobalDataRelated Reports
GlobalData (2018). Glioblastoma – Opportunity Analysis and Forecast to 2027, To Be Published





Related Company Profiles
Bristol-Myers Squibb Co
GBM SRL
BMS AS