Merck’s trial of Keytruda regimen fails to meet primary endpoint
The randomised, double-blind study involved 1,095 patients to assess adjuvant treatment with Keytruda plus chemotherapy.

The randomised, double-blind study involved 1,095 patients to assess adjuvant treatment with Keytruda plus chemotherapy.


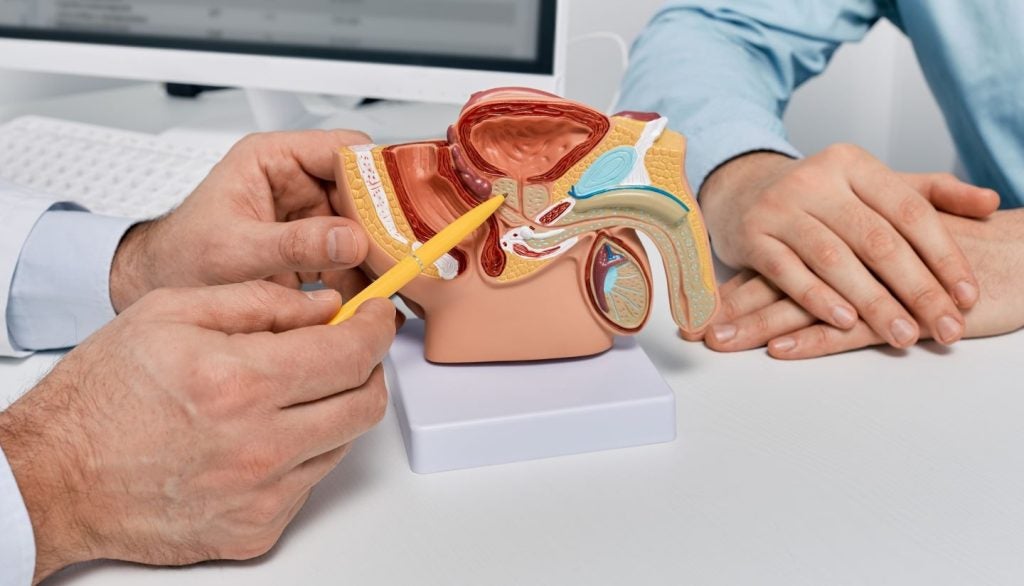
The FDA granted de novo classification to the Symani Surgical System for soft tissue manipulation in microsurgery in April 2024.
The pharmaceutical industry continues to be a hotbed of patent innovation. Activity is driven by the evolution of new treatment...


Pharma Technology Focus is our digital magazine, free to read online on all devices. Click the magazine cover to read the latest issue. You can subscribe for free to have each new issue delivered to your inbox.
“Defensibility” key for AI-centric small biotechs seeking investment
Klineo wins €2m to advance AI patient recruitment platform
Hyperfine starts enrolment in portable imaging workflow study
Acarix starts clinical workflow study for its AI-powered cardiac diagnostic