Pharmaceutical company Immunicum has started dosing patients in the Phase Ib/II Iliad clinical trial of ilixadencel and checkpoint inhibitors (CPIs) combination to treat cancer.
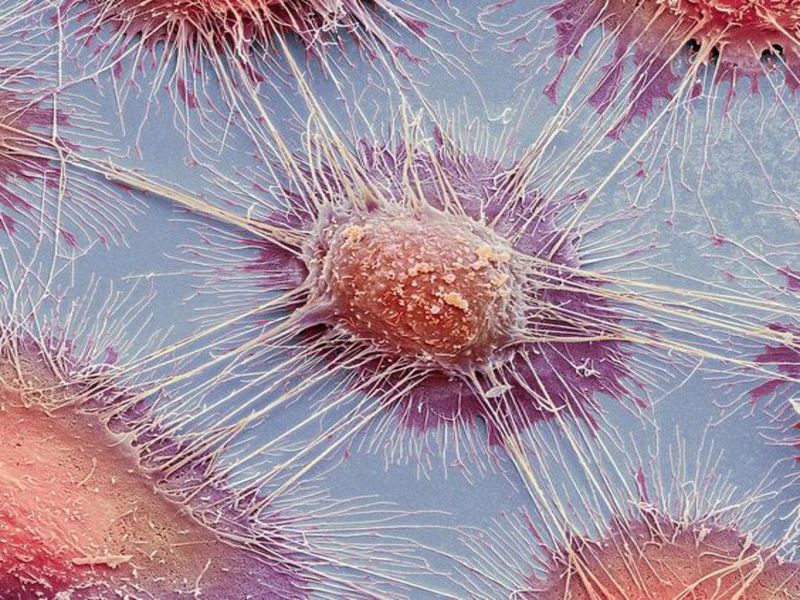
Ilixadencel is a cell therapy candidate designed as an off-the-shelf cancer immune primer using activated allogeneic dendritic cells obtained from healthy blood donors.
Intratumoral injection of dendritic cells is said to produce an inflammatory response that causes tumour-specific activation of the patient’s cytotoxic T-cells.
The open-label, multicentre Phase Ib/II trial will assess the combination therapy’s safety and efficacy in patients with head and neck squamous cell carcinoma (HNSCC), non-small cell lung cancer (NSCLC) or gastric and gastroesophageal junction adenocarcinoma (GA/GEJ).
Immunicum chief medical officer Peter Suenaert said: “The start of the ILIAD clinical trial is a positive and important step in the development of ilixadencel, testing its ability to prime a patient’s immune system to fight the cancer.
How well do you really know your competitors?
Access the most comprehensive Company Profiles on the market, powered by GlobalData. Save hours of research. Gain competitive edge.

Thank you!
Your download email will arrive shortly
Not ready to buy yet? Download a free sample
We are confident about the unique quality of our Company Profiles. However, we want you to make the most beneficial decision for your business, so we offer a free sample that you can download by submitting the below form
By GlobalData“This study will give us the opportunity to further evaluate its potential as a backbone component in combination therapies to treat solid tumors, and we look forward to exploring the synergistic effects of the immune activity of ilixadencel together with CPIs.”
The Phase Ib part of ILIAD will evaluate the safety and determine the optimal dose and schedule of ilixadencel in combination with the standard dose of CPI pembrolizumab (Keytruda) in 21 patients.
This portion will be carried out at clinical sites across the US.
The trial’s Phase II part will involve three randomised, controlled studies to investigate the safety and efficacy of ilixadencel plus CPI combination in a total of up to 150 patients in the US and Europe.
In NSCLC patients, ilixadencel will be combined with pembrolizumab (Keytruda), while HNSCC and GA/GEJ patients will be administered with ilixadencel and avelumab (Bavencio) combination.
Merck and Pfizer will supply Keytruda and Bavencio, respectively, under a collaboration agreement.
Sweden-based Immunicum secured protocol approval from the US Food and Drug Administration (FDA) for the ILIAD trial in July last year.







