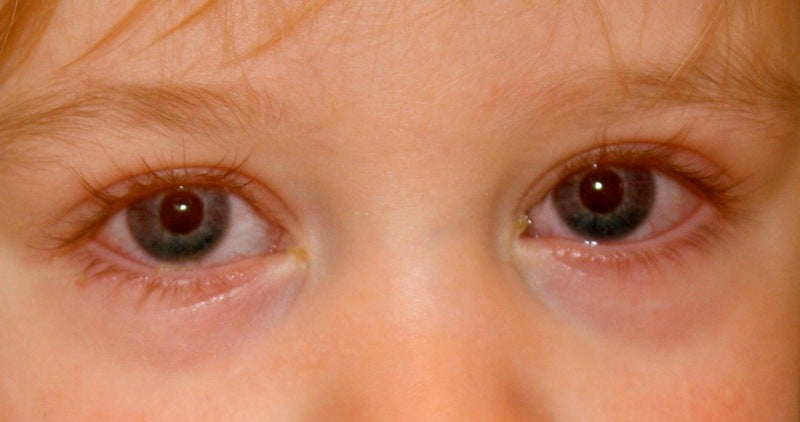
Okogen has commenced the Phase II RUBY clinical trial to investigate the safety and efficacy of OKG-0301 for the treatment of acute adenoviral conjunctivitis.
Adenoviral conjunctivitis is a highly contagious disease and one of the major causes of eye infections across the globe.
The RUBY trial includes a multicentre, randomised, placebo-controlled, double-masked design and will be carried out at six to seven sites in Australia.
It is expected to assess multiple doses of OKG-0301 in 219 adult patients with acute adenoviral conjunctivitis.
University of Sydney Save Sight Institute professor Stephanie Watson is the principal investigator of the trial.
How well do you really know your competitors?
Access the most comprehensive Company Profiles on the market, powered by GlobalData. Save hours of research. Gain competitive edge.

Thank you!
Your download email will arrive shortly
Not ready to buy yet? Download a free sample
We are confident about the unique quality of our Company Profiles. However, we want you to make the most beneficial decision for your business, so we offer a free sample that you can download by submitting the below form
By GlobalDataOkogen aims to examine the capability of OKG-0301 in lowering viral burden, which is important in limiting the extent of the disease and stopping the spread of virus.
The company is also expected to evaluate the safety of OKG-0301 and the potential of OKG-0301 to reduce longer-term complications of adenoviral infection, which negatively affect vision and result in scarring of the ocular surface.
Okogen CEO Brian Strem said: “Reducing the highly contagious nature of this infection while accelerating the resolution of symptoms has been a long-sought goal of the ophthalmology community.
“The existing body of OKG0301 data suggests that this innovative antiviral agent can achieve both of these clinically important outcomes.
“We are excited to initiate this Phase II trial and expect to complete enrollment by the end of 2019.”
OKG-0301 is a topical ophthalmic formulation of ranpirnase, a ribonuclease (RNase) that contains broad-spectrum antiviral properties.








