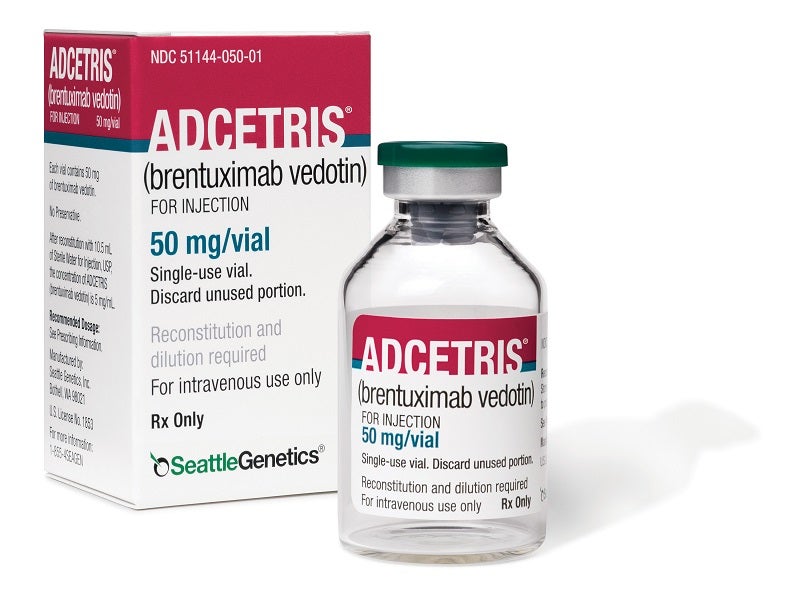
Adcetris® (brentuximab vedotin) is an antibody drug conjugate indicated for the treatment of relapsed or refractory (r/r) Hodgkin’s lymphoma (HL), anaplastic large cell lymphoma (ALCL) and peripheral T-cell lymphomas.
The drug was developed by Seattle Genetics and Millennium Pharmaceuticals, a wholly owned subsidiary of Takeda Pharmaceutical.
In February 2011, Seattle Genetics filed a biologics licence application (BLA) with the US Food and Drug Administration (FDA) for the drug. The FDA accepted the BLA in May 2011.
A six-month priority review was also granted for the r/r HL and ALCL indications, and the drug was approved in August 2011 for the treatment of HL and systemic anaplastic large cell lymphoma (sALCL).
Takeda and Millennium submitted a marketing authorisation application (MAA) for the drug to the European Medicines Agency (EMA), which included Phase II trial data. The MAA was accepted in June 2011.
Adcetris for injection is available as a sterile, white to off-white, preservative-free lyophilised cake or powder in single-use vials.
Regulatory approval for Adcetris
In July 2012, the EMA gave Adcetris a positive opinion for conditional approval for the treatment of adult patients with r/r CD30-positive HL and r/r sALCL.
The drug also obtained orphan drug designation for both the r/r HL and ALCL indications in Europe.
Adcetris was approved in Japan by the Ministry of Health, Labour and Welfare (MHLW) for the treatment of ALCL patients in January 2014.
In August 2015, the FDA granted approval to Adcetris for the treatment of classical HL patients at high risk of relapse or progression after autologous stem cell transplant (ASCT). The FDA’s approval was based on positive results from Aethera, a Phase III clinical trial.
In January 2016, the European Commission (EC) approved Adcetris for re-treating adult patients with r/r HL or sALCL who had previously received and responded to Adcetris before subsequently relapsing. In June 2016, the EC approved the drug to treat CD30-positive HL in patients at high risk of relapse or progression after ASCT.
Adcetris was approved in Japan in September 2018 for treating CD30-positive frontline HL patients in combination with doxorubicin, vinblastine and dacarbazine (AVD). The MHLW’s approval was based on positive results from the Phase III trial ECHELON-1.
In November 2018, Adcetris, combined with cyclophosphamide, doxorubicin and prednisone (CHP) chemotherapy, received FDA approval for the treatment of patients previously untreated with sALCL or other CD30-expressing peripheral T-cell lymphomas (PTCL). This approval was based on results from the Phase III clinical trial ECHELON-2.
China’s Centre for Drug Evaluation granted priority review to Adcetris in June 2019.
In December 2019, Adcetris obtained approval for peripheral T-cell lymphomas in Japan. The drug was also approved for additional dosage and administration in paediatric patients with r/r HL and peripheral T-cell lymphomas.
In March 2020, the European Medicines Agency’s (EMA) Committee for Medicinal Products for Human Use (CHMP) granted a positive opinion to Adcetris for the treatment of previously untreated sALCL in combination with CHP.
In May 2020, China’s National Medical Products Administration (NMPA) approved Adcetris (brentuximab vedotin) for the treatment of adult patients with r/r sALCL or CD30-positive Hodgkin lymphoma. That year, the EC also extended the existing conditional marketing authorisation of Adcetris to include previously untreated sALCL in combination with CHP.
Adcetris is currently being marketed in more than 70 countries or regions for r/r HL and sALCL.
Adcetris (brentuximab) was developed by Seattle Genetics.
Hodgkin’s lymphoma symptoms
Lymphoma is a type of blood cancer occurring in white blood cells. HL and Non-HL are the two types of the disease.
White blood cells are made up of T-lymphocytes (T-cells) and B-lymphocytes (B-cells), which provide immunity against bacterial infections and viruses respectively. Increased and abnormal growth in the number of lymphocytes results in lymphoma.
HL is usually observed in young people aged 15 to 35 years or adults aged older than 50 years.
Adcetris mechanism of action
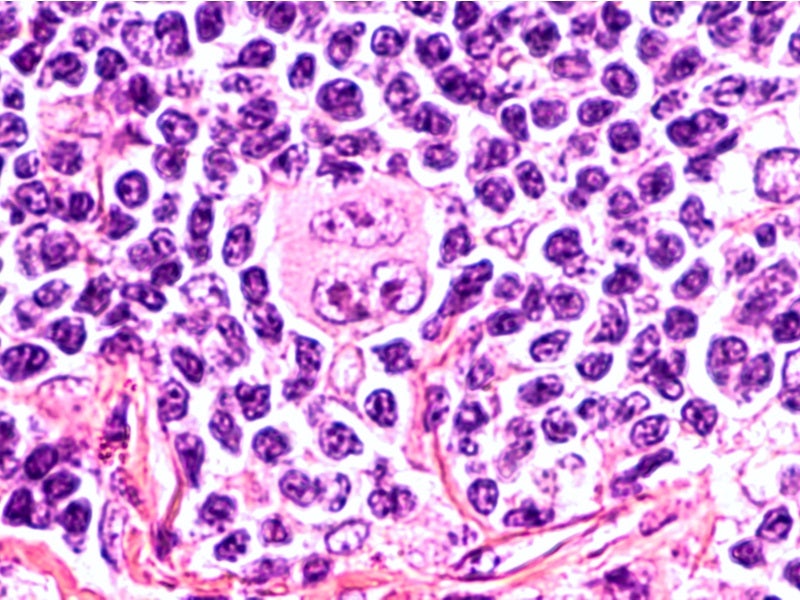
Hodgkin Reed-Sternberg cells are present in HL patients’ white blood cells, which express a protein called CD30.
Adcetris is an antibody-drug conjugate (ADC) consisting of monomethyl auristatin E (MMAE) linked to the monoclonal antibody anti-CD30. The ADC binds to the CD30 in Reed-Sternberg cells and forms an ADC-CD30 complex.
This complex moves towards the lysosome where the MMAE is released, which causes the microtubules to break down, resulting in G2/M cell cycle arrest and cell death (apoptosis).
Clinical trials of Adcetris on Hodgkin’s lymphoma patients
Phase I trials to evaluate Adcetris’ safety and efficacy on the cardiac system in patients with CD30-positive cancer began in January 2010. The following month, Phase I trials to evaluate the drug’s safety and efficacy when used in combination with chemotherapy for treating newly diagnosed HL patients were initiated.
A total of 70 patients were enrolled and given brentuximab in combination with chemotherapy with doxorubicin, bleomycin, vinblastine, dacarbazine in combination (ABVD), or brentuximab in combination with AVD. The study was completed by November 2012.
A Phase II trial was conducted to evaluate brentuximab’s safety and efficacy as a single agent in patients with r/r HL. The study began in February 2009 and reached primary completion in August 2010.
A Phase III trial called Aethera was conducted to evaluate the drug’s safety and efficacy in comparison with placebo. A total of 329 post-ASCT patients were enrolled in the randomised, double-blind, placebo-controlled study.
All patients in the group were at high risk of residual HL following a stem cell transplant. They were administered either brentuximab or placebo intravenously every 21 days during the trial.
The study’s primary endpoint was median progression-free survival (PFS). A median PFS of 42.9 months was achieved for patients treated with Adcetris, compared to 24.1 months for patients on placebo. The study began in April 2010 and was completed by June 2013.
Clinical trials on anaplastic large cell lymphoma patients
Phase I trials to evaluate brentuximab’s safety in combination with chemotherapy in ALCL patients began in February 2011. The trial enrolled 52 patients and was completed by December 2012.
Another Phase II trial was carried out to evaluate the drug’s safety and efficacy as a single agent in ALCL patients, with 58 patients enrolled. The trial began in February 2009 and had a primary completion date of August 2010. The patients were given 1.8mg/kg of brentuximab every 21 days.
Another Phase II trial to assess brentuximab’s efficacy in controlling ALCL, lymphomatoid papulosis or mycosis fungoides began in June 2011 and enrolled 35 patients, who were administered 1.8mg/kg of brentuximab a day intravenously.
Adcetris, in combination with chemotherapy, was studied in a global, randomised, double-blind, multi-centre Phase III trial, ECHELON-2, in previously untreated patients with CD30-expressing PTCL. Significant improvement was noticed in the progression-free survival of patients given Adcetris compared to those on standard-of-care therapy.