Victrelis (boceprevir) is an oral HCV protease inhibitor indicated for the treatment of chronic infection with hepatitis C (HCV) that is currently in development.
The drug was developed by Merck; it was originally being developed by Schering-Plough, which Merck acquired in 2009.
In October 2010, Merck announced final clinical results from two Phase III trials of Victrelis. The company submitted a new drug application in the US and marketing authorisation application in the EU for Victrelis in 2010.
The US Food and Drug Administration (FDA) approved the new drug application in January 2011 with priority review status. The European Medicines Agency also accepted the marketing authorisation application submitted by the company for accelerated assessment of the drug.
In April 2011, the FDA’s Antiviral Drugs Advisory Committee recommended the approval of the drug, and it was finally approved on 13 May 2011.
On 20 May 2011, the European Medicines Agency’s Committee for Medicinal Products for Human Use issued a positive opinion for the approval of the drug as a treatment for chronic HCV. The drug obtained European approval in July 2011.
Health Canada approved Victrelis in August 2011.
Few treatment options for HCV infection
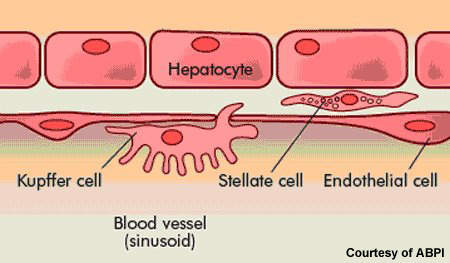
HCV is an infection of the liver caused by the hepatitis C virus. It is most commonly transmitted by blood-to-blood contact. Before routine screening for HCV, people were at risk of contracting HCV infection through the use of contaminated blood products in blood transfusions (post-transfusion hepatitis). It is common in HIV positive patients: about a third of all HIV patients are co-infected with HCV.
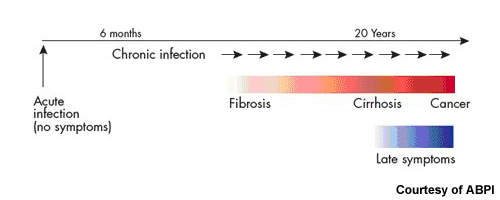
While some people infected with HCV spontaneously clear the infection, for the majority of patients HCV is a chronic disease. Current treatment options for patients with chronic HCV are limited. At present, standard treatment of HCV infection usually consists of oral ribavirin, a nucleoside analogue, in combination with a pegylated interferon, which is administered by injection.
The aim is to achieve a sustained virological response (SVR), in which HCV is no longer detectable in the blood for six months after the cessation of treatment. Most patients undergo treatment for an initial 12 weeks, with responders often continuing for periods of up to 48 weeks. Patients with HIV/HCV co-infections may require even longer periods of treatment.
Because many patients with chronic HCV infection fail to respond to current therapy, or respond only poorly, there is a need for new and improved drugs to treat this serious and potentially fatal liver disease.
Direct antiviral therapy for HCV
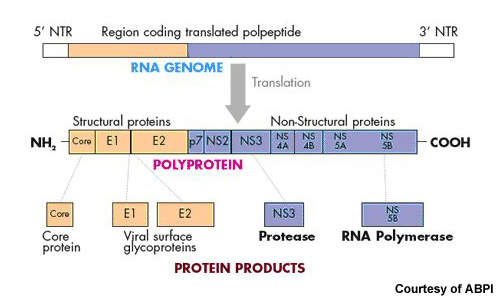
Currently approved treatments for chronic HCV infection are designed to boost the host’s immune response to help eradicate the virus. In contrast, Victrelis is designed to directly attack the virus by inhibiting protease enzymes that are critical to HCV replication. Clinical studies suggest that it is a potent addition to standard therapies in patients with HCV genotype-1, the most common and hardest form of HCV to treat.
In HCV SPRINT-1, a Phase II study in 595 treatment-naïve patients with chronic HCV genotype 1, treatment with Victrelis improved viral clearance rates in comparison with standard therapy alone. In this 48-week study, SVR rate at 12 weeks after the end of treatment was 74% in patients who received four weeks of peginterferon alfa-2b and ribavirin before the addition of Victrelis 800mg TID (standard therapy lead-in), compared to 38% for patients receiving 48 weeks of standard therapy alone.
SVR at 12 weeks was 66% in patients who received 48 weeks of Victrelis in combination with peginterferon alfa-2b and ribavirin from the beginning of treatment (standard therapy lead-in).
For patients who received the standard therapy lead-in and experienced a rapid virological response (undetectable HCV-RNA in plasma after four weeks of treatment), SVR was 82% in the 28-week regimen and 92% in the 48-week regimen.
Victrelis also demonstrated efficacy in HCV patients who failed to respond to prior treatment with peginterferon alfa-2b/ribavirin combination therapy. These so-called “null nonresponders” constitute the most difficult to treat HCV patient population.
Phase III development
On the back of successful Phase II studies, Victrelis progressed to Phase III development, where it was studied in combination with peginterferon alpha-2b and ribavirin.
One trial focused on treatment-naive patients (HCV SPRINT-2), while the second enrolled patients who had failed previous treatments including both relapsers and non-responders (HCV RESPOND-2). The two tests were randomised, double-blind, placebo-controlled trials.
Schering completed enrolment of patients for SPRINT-2 in January 2009. Patient enrolment for RESPOND-2 was completed in November 2008. About 1,500 patients were enrolled in centres across the US and other international sites.
The drug met the primary endpoints of HCV SPRINT-2 and HCV RESPOND-2 in August 2010. In both studies it was found that Victrelis in combination with peginterferon alpha-2b and ribavirin produced undetectable virus levels in patients with chronic HCV genotype 1 infection.
The final results of the two Phase III clinical trials were announced in October 2010. They demonstrated that Victrelis achieved higher SVR rates in adult patients who previously experienced failed treatment or were new to the treatment for chronic HCV genotype 1 in comparison to the control.
A Phase III trial in Russian patients for the treatment of HCV genotype 1 will be initiated in November 2011. The trial will evaluate the efficacy of Victrelis in combination with peginterferon alfa 2-b and ribavirin. Around 210 patients are expected to be enrolled for the study which is scheduled for completion by 2014.
Marketing commentary
Estimates from the WHO suggest that as much as 3% of the world’s population has HCV, 80% of whom are chronically infected. It is a major cause of liver cirrhosis and liver cancer.
Current drug therapy offers benefit to some patients with chronic HCV infection: about 30–50% of patients respond to combination peginterferon alpha-2b/ribavirin therapy. Nonetheless, a large treatment gap remains for which HCV protease inhibitors represent a potentially important new class of drugs.
In May 2011, Merck signed several strategic agreements with Roche to spread awareness about the diagnosis and treatment methods of chronic HCV among physicians and patients across the world. As per the agreements, new treatment options will be explored using combinations of marketed and investigational drugs of both the companies. In addition, Roche will encourage the use of Victrelis by physicians globally, beginning with the US.