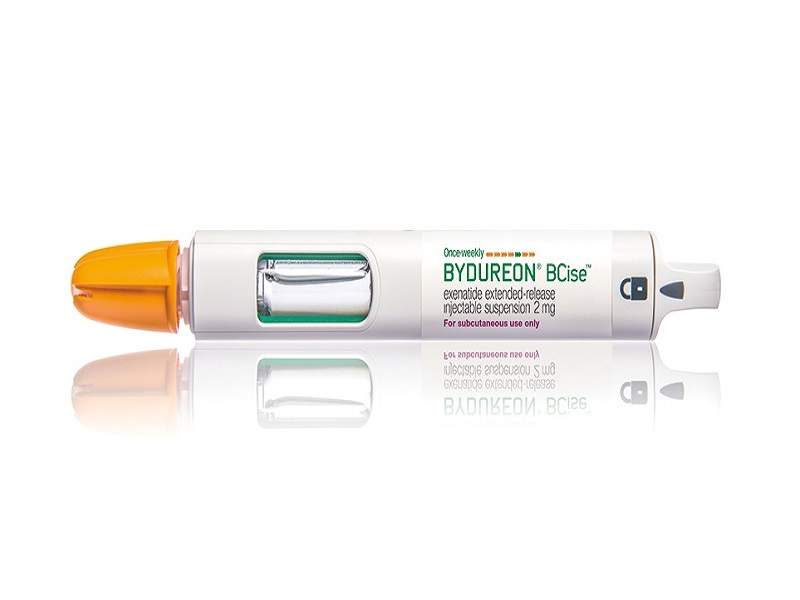
Bydureon® BCise™ (exenatide extended-release) is an injectable suspension containing a glucagon-like peptide-1 (GLP-1) receptor agonist.
Developed by AstraZeneca, Bydureon® BCise™ was approved by the US Food and Drug Administration (FDA) in October 2017 for the treatment of Type-2 diabetes in adults.
The drug is indicated for patients whose blood sugar remains uncontrolled on one or more medications in addition to diet and exercise.
Type-2 diabetes causes and symptoms
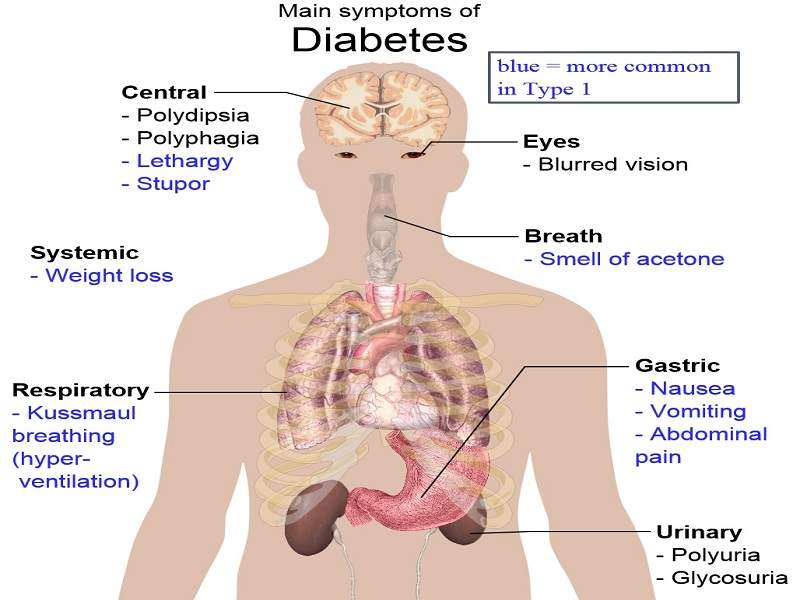
Diabetes is a metabolic disorder that results in high blood glucose levels. Type-2 diabetes is the most common form of the disease, wherein the body does not utilise insulin efficiently.
It can lead to the onset of health problems, including heart disease, strokes, diabetic retinopathy and kidney failure.
The most common symptoms associated with Type-2 diabetes include increased thirst, frequent urination, unusual weight loss, fatigue and increased hunger. These symptoms are found in between 85% and 95% of adult patients diagnosed with diabetes.
Type-2 diabetes is estimated to affect approximately 27 million people in the US.
Bydureon® BCise™’s mechanism of action
Bydureon® BCise™ contains a glucagon-like peptide-1 (GLP-1) receptor agonist, which works by enhancing glucose-dependent insulin secretion by the pancreatic beta-cell, and restraining inappropriately elevated glucagon secretion. The drug also slows down gastric emptying.
The drug is available as a 2mg suspension in a pre-filled single-dose auto-injector device, which can be administered once a week.
Clinical trials on Bydureon BCise
The FDA’s approval for Bydureon® BCise™ was based on results obtained from two Phase III clinical trials.
The first trial was a 28-week randomised, open-label comparator-controlled trial that compared the safety and efficacy of Bydureon® BCise™ with Byetta. It enrolled 375 patients with Type-2 diabetes and inadequate glycaemic control.
The primary endpoint of the study was change in HbA1c from baseline at week 28. Results from the clinical trial demonstrated that patients treated with Bydureon® BCise™ 2mg once-weekly dose achieved a statistically significant reduction in HbA1c compared to Byetta 10mcg twice daily.
The study also found that Bydureon® BCise™ was statistically superior to Byetta 10mcg twice daily.
The second Phase III clinical trial compared the safety and efficacy of Bydureon® BCise™ with sitagliptin and placebo as an add-on to metformin. This open-label comparator and placebo-controlled trial enrolled 364 patients with Type-2 diabetes, whose glycaemic control was inadequate with metformin therapy.
The primary endpoint of the second study was also change in HbA1c from baseline at week 28. Results from the study showed that the patients treated with Bydureon® BCise™ achieved a statistically significant mean reduction in HbA1c compared to placebo.
The study also found that Bydureon® BCise™ was statistically superior to placebo.
The most common adverse reaction reported was nausea.
Marketing commentary on AstraZeneca
AstraZeneca is a global biopharmaceutical company involved in the discovery, development and commercialisation of drugs for the treatment of diseases in the therapy areas of oncology, cardiovascular, metabolic and respiratory.
The company has facilities across 100 countries, which develop treatments for autoimmunity, neuroscience and infection.
Bydureon® BCise™ was launched to the US market in January 2018. Other medications available in the market for the same indication include Byetta developed by Amylin Pharmaceuticals/ Eli Lilly, and Trulicity (dulaglutide) developed Eli Lilly and Company.