Dulera (mometasone furoate and formoterol fumarate dihydrate) is a new fixed-dose combination inhaler for the treatment of asthma. It was developed by Schering-Plough, which was merged with Merck in November 2009.
In June 2010, dulera was approved by the US Food and Drug Administration (FDA) for treating asthma in patients who are 12 years of age or older. Merck is currently conducting Phase III clinical trials on dulera for chronic obstructive pulmonary disease (COPD).
Causes of asthma and statistics for the chronic lung disease
Asthma is a chronic lung disease which affects the airways. These airways are tubes that carry air in to and out of the lungs. The disease causes swelling and narrowing of the airways, causing difficulty in breathing.
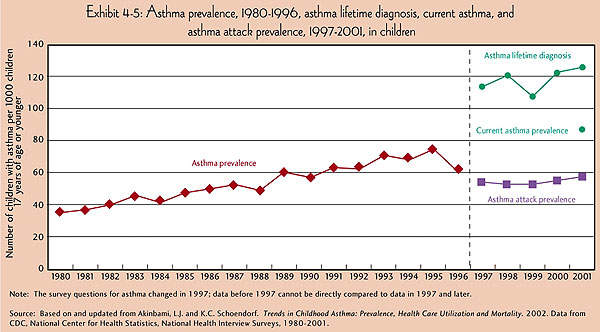
It affects people of all ages, but more commonly occurs during the childhood. About 22 million people in the US alone are estimated to have asthma, and six million of them are children.
Dulera’s mechanism of action and symptom prevention
The drug contains a combination product of corticosteroids (mometasone furoate) and long-acting beta2-adrenergic agonists (LABA) (formoterol fumarate). It prevents symptoms of wheezing and shortness of breath in asthma patients.
The mometasone furoate contained in the drug reduces the irritation and swelling of the airways, while the formoterol fumarate works by opening airways in the lungs to make breathing easier. The drug is administered through oral inhalation.
Clinical trials on Merck’s drug / medication for COPD conditions

Dulera (mometasone furoate and formoterol fumarate dihydrate) is a new fixed-dose combination inhaler for the treatment of asthma.
Phase I clinical trial on dulera was conducted between June 2010 and March 2011. It was a randomised, single-blind efficacy study. It enrolled 16 patients aged between 18 and 55 years of age, who were suffering from mild to moderate asthma.
The primary outcome measure of the study was to find the change in FEV1 (a calculated ratio used in the diagnosis of obstructive and restrictive lung disease) and change in plasma. The secondary outcome measures included finding allergen-induced changes in urinary leukotriene E4 (LTE4) and sputum.
Related project
Surfaxin (Lucinactant) – Treatment for Preventing Infant RDS
Surfaxin (Lucinactant) is a non-pyrogenic pulmonary surfactant used for the prevention of respiratory distress syndrome (RDS) in premature infants.
A Phase II clinical trial on dulera was conducted between December 2010 and October 2011. It was a randomised, evaluator-blind, crossover and single dose study, which was focused towards finding the effectiveness of formoterol fumarate (FF) in combination with a mometasone furoate (MF) metered dose inhaler (MDI), delivered with and without a spacer versus placebo and Foradil Aerolizer.
The primary outcome of the study was finding the changes in FEV1 from baseline after a single dose of MF/F MDI 100/10 mcg with spacer versus placebo MDI. The secondary outcome measure included finding the change in FEV1 from the baseline by comparing MF/F MDI 100/10 mcg with a spacer versus MF/F MDI 100/10 mcg without a spacer.
FDA approval for dulera was based on two randomised Phase III clinical trials. The trials recruited a total of 1,509 patients with asthma, who were aged 12 years and above.
The first Phase III trial compared dulera 100mcg/5mcg to mometasone furoate 100mcg, formoterol 5mcg and placebo. The second Phase III clinical trials compared dulera 200mcg/5mcg with dulera 100mcg/5mcg and mometasone furoate 200mcg. In both the studies, the patients were administered with the drugs twice daily using metered dose inhalers.
The results from the Phase III clinical trials showed that patients administered with dulera experienced significant improvement from baseline in lung function at week 12 when compared to mometasone furoate in trials I and II and to placebo in trial I.
Dulera marketing commentary and competition from GSK and AZN
Dulera has been commercially available in the US market since July 2010. Other combination drugs approved by the FDA for the same indication include salmeterol and fluticasone (advair) developed by GSK, along with formoterol and budesonide (symbicort) developed by AstraZeneca.
Related content
Spiriva – Treatment for Chronic Obstructive Pulmonary Disease, United States of America
Spiriva is an anticholinergic drug targeted at chronic obstructive pulmonary disease (COPD).
Kalydeco (Ivacaftor) – Treatment for Cystic Fibrosis
Kalydeco (Ivacaftor / VX-770) is a cystic fibrosis transmembrane conductor regulator (CFTR) potentiator indicated for the treatment of cystic fibrosis (CF).