Enbrel is an immunosuppressant that inhibits the production of tumour necrosis factor (TNF) in patients suffering from rheumatoid arthritis (RA). RA is a chronic, progressive inflammatory disorder that primarily affects the joints and is estimated to affect about 2.9 million people in the US.
Enbrel is co-marketed by Wyeth Pharmaceuticals and Amgen in North America. Outside North America, Enbrel is sold by Wyeth alone. In October 2009, Wyeth was acquired by Pfizer. In Japan the drug is marketed by Takeda Pharmaceuticals.
Enbrel became the first biologic medicine to be approved by the US Food and Drug Administration (FDA) in November 1998 for the treatment of moderate to severe RA. Since its approval for RA, the drug has also been approved for treatment of juvenile idiopathic arthritis (JRA), psoriatic arthritis, ankylosing spondylitis (AS) and moderate to severe plaque psoriasis.
In February 2000, Enbrel was approved by the European Medicines Evaluation Agency (EMEA) for treating juvenile chronic arthritis (JCA).
Enbrel was also approved by Health Canada for treating moderate to severe active adult RA in December 2000.
Rheumatoid arthritis
The immune system is responsible for protecting the human body against foreign invaders such as bacteria, fungi and viruses. One of the ways in which the immune system defends the body is by increasing the flow of blood and immune cells to an affected part of the body, causing inflammation. The inflammation process involves chemical messengers including TNF.
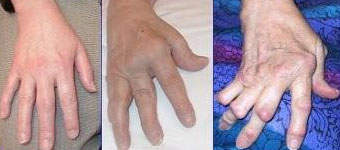
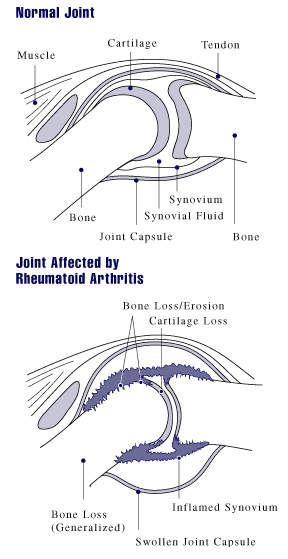
In those suffering from RA, TNF is produced in excess leading to chronic inflammation. As the inflammation increases, the synovium (a thin lining of the joints) thickens, leading to the destruction of articular cartilage (tissue that covers the joints) and causing ankylosis (stiffness) of the joints.
The exact cause of RA is unclear but autoimmunity determines the chronicity and progression of the disease. RA can strike at any age but normally develops between the ages of 25 and 50 and affects more women than men. Even without severe symptoms, RA causes serious disability leading to significant loss of functioning and mobility and irreversibly destroying the joints. RA can also produce inflammation in the lungs, pericardium (membrane surrounding the heart), pleura (layer protecting the lungs), and sclera (white outer wall of the eye).
Enbrel inhibits production of TNF
Enbrel is a type of protein similar to the natural proteins occurring in the body. It is made by fusing two naturally found soluble human TNF receptors to form a large molecule weighing 150kDa. Enbrel binds with TNF molecules and stops them from binding with the TNF receptor sites. By binding with TNF, Enbrel renders the TNF molecules inactive, which results in reducing the inflammation and progressive deterioration caused by TNF.
By inhibiting production of TNF, however, Enbrel lowers the immune system’s ability to fight infections. Using Enbrel has led to severe side effects including TB, multiple sclerosis, seizures, heart failure, allergic reactions, lymphoma and death.
Clinical trials for RA and other types of arthritis
Clinical trials for Enbrel began in 1992. In clinical studies, Enbrel exhibited its ability to reduce pain and duration of morning stiffness and improve swollen and tender joints. It helped in enabling patients to participate in daily activities.
Phase I and Phase II studies were conducted in 1993–94 for RA and positive results from Phase II studies were revealed in 1995. Phase III studies with 632 patients tested the ability of Enbrel for one year to slow the progression of RA. Positive results from Phase III studies were announced in 1997. Phase III studies revealed that Enbrel showed marked improvement in patients with RA symptoms such as joint pain and joint swelling.
Enbrel has also been studied in patients suffering from JRA, psoriatic arthritis, AS and moderate to severe plaque psoriasis. Enbrel was approved for treatment of JRA in May 1999, for psoriatic arthritis in 2002, for AS in 2003 and for moderate to severe plaque psoriasis in 2004.
More recent ongoing studies include the Comet study, which has been designed to compare the safety and efficacy of combining Enbrel with Methotrexate and Methotrexate alone in patients suffering from early RA. Early results from the study have revealed that patients taking Enbrel in combination with Methotrexate are able to work for longer.
Marketing commentary
With a growing number of people being diagnosed with RA, Enbrel is one of the first breakthroughs achieved in treating the disease. Sales of Enbrel have been growing steadily and the drug has continued to maintain a leading position in the rheumatology and dermatology segments. In the second quarter of 2009, Enbrel recorded $899m in sales, a 7% increase from $841m in 2008.