Erbitux is a chimaeric monoclonal antibody (MAb) that is specific for the epidermal growth factor receptor (EGFR). A new cancer biotherapy developed by US biopharmaceutical company ImClone Systems, Erbitux is an approved treatment for metastatic colorectal cancer and locally advanced squamous cell carcinoma of the head and neck.
Bristol-Myers Squibb (BMS), which plans to acquire ImClone Systems, has marketing rights to Erbitux in North America, while Merck KGaA (now Merck-Serono) has rights to the drug outside the US and Canada as well as joint rights with BMS in Japan.
Inhibition of EGFR
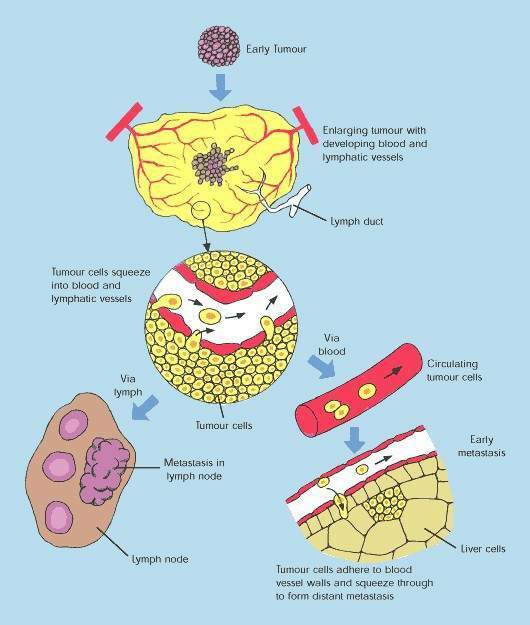
Over-expression of EGFR is common in many solid tumours, such as colorectal and lung carcinomas as well as cancers of the head and neck. It correlates with increased metastasis, decreased survival and a poor prognosis.
EGFR protects malignant tumour cells from the cytotoxic effects of chemotherapy and radiotherapy, making these treatments less effective.
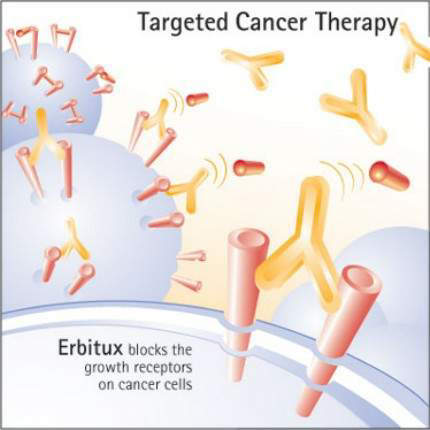
Erbitux binds to the extracellular domain of EGFR on the tumour cell, thereby inhibiting receptor-associated tyrosine kinase. This inhibition blocks the intracellular pathways associated with tumour cell proliferation, so preventing tumour growth and dissemination as well as inducing cell death (apoptosis).
By interfering with cell signalling pathways involved in cell proliferation, inhibition of EGFR-associated tyrosine kinase represents a novel approach to the treatment of solid tumours. Erbitux is one of several cancer drugs that target EGFR.
Potential in advanced NSCLC
Beyond its currently licensed indications, Erbitux is also being investigated as a potential treatment for other solid tumours including advanced non-small cell lung cancer (NSCLC).
Encouraging results have been demonstrated in two large-scale, phase III trials in which Erbitux was administered in combination with standard chemotherapy to patients with advanced NSCLC, for whom prognosis is often very poor.
In the 1,125-patient pivotal FLEX (First-line in Lung cancer with ErbituX) multinational phase III study, in which Erbitux was combined with vinorelbine and cisplatin, a statistically significant improvement in overall survival was observed in the three-drug treatment arm. Median overall survival was 11.3 months in patients receiving Erbitux compared with 10.1 months in those receiving chemotherapy alone (p=0.044).
A trend towards increased survival was also seen in the open-label BMS-099 trial, although the difference between treatment arms didn’t reach statistical significance (median overall survival 9.7 months with Erbitux vs 8.4 months; p=0.17). In this trial Erbitux was added to standard therapy with a taxane and carboplatin.
Erbitux progresses to phase III trials in gastric cancer
Gastric, or stomach cancer as it more commonly known, is often diagnosed late when treatment options are limited. Most patients with advanced gastric cancer receive palliative chemotherapy.
Phase II studies of Erbitux in combination with standard chemotherapy in patients with advanced gastric cancer suggest that it may provide additional clinical benefit. On the back of these findings, phase III clinical trials have been initiated. Patient enrolment is now underway in the open-label EXPAND (Erbitux in combination with Xeloda and cisplatin in advanced esophago-gastric cancer) trial.
This 800-plus patient study will investigate the efficacy and safety of Erbitux in combination with cisplatin and capecitabine as first-line treatment for metastatic gastric adenocarcinoma including gastroesophageal junction adenocarcinoma. Progression-free survival will be the primary endpoint.
Marketing commentary
Despite its difficult path to market following FDA rejection of the early phase II data in colorectal cancer, Erbitux finally secured approval as a treatment for metastatic colorectal cancer in both the US and Europe in 2004. It was subsequently approved for locally advanced squamous cell carcinoma of the head and neck, where Erbitux offers the prospect of improved disease control and extended survival when combined with radiotherapy.
As new phase III data suggests, Erbitux could be a potentially useful addition to standard chemotherapy for the treatment of advanced NSCLC. It is believed that BMS and ImClone are in discussion with the FDA about filing Erbitux for first-line treatment of advanced NSCLC.