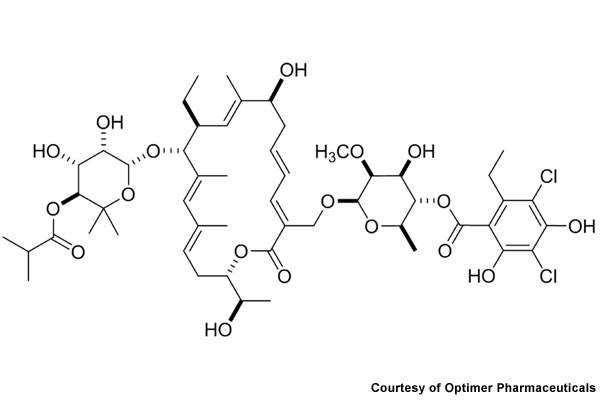
Dificid (fidaxomicin) is the first in a new class of antibiotics developed by Optimer Pharmaceuticals to treat Clostridium difficile infections. The drug was formerly known as OPT-80, PAR-101 and Difimicin.
Based on the results of successful Phase III clinical trials, Optimer submitted a new drug application to the US Food and Drug Administration (FDA) for tghe drug in December 2010.
The FDA accepted the application in January 2011, and also awarded Fidaxomicin orphan drug designation. Optimer’s request for a six-month priority review was also accepted.
In May 2011, the FDA approved Dificid for the treatment of Clostridium difficile-associated diarrhoea in people aged 18 years and older.
Targeting Clostridium dfficile
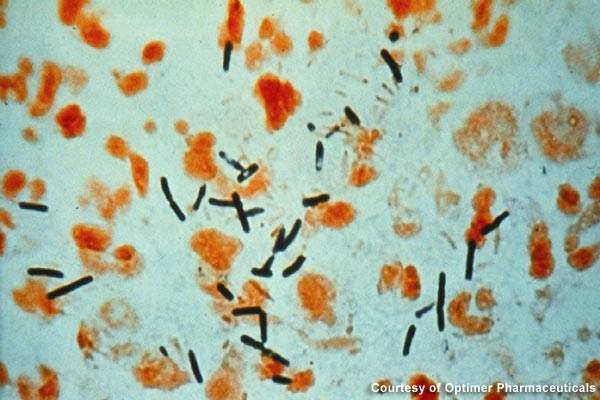
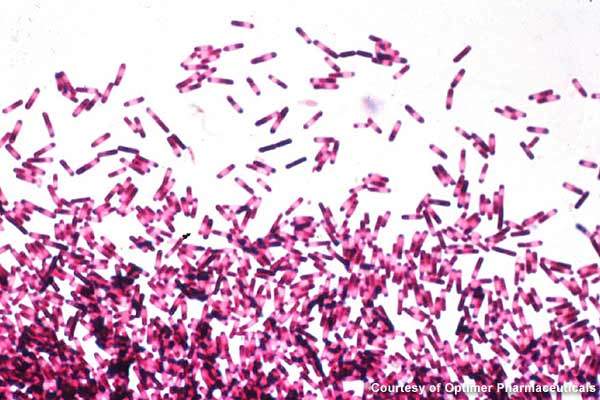
Clostridium dfficile infections are largely spread in hospitals, long-term healthcare facilities and community settings. The gram-positive bacteria creates severe infection in the colon, resulting in severe diarrhoea, colon inflammation and, in extreme cases, death.
In 2007, the disease was believed to have led to over 8,000 deaths in the UK. Broad-spectrum antibiotics that destroy the normal gut flora of humans are the prime reason for the growth of C. difficile.
Elderly people with weak immune systems, or people who have had an extended stay in a healthcare environment, are vulnerable to the disease. Treatment options for C. difficile. include oral Vancocin, the only US Food and Drug Administration (FDA)-approved therapeutic option and off-label use of metronidazole. Approximately 20–30% of patients who had responded to these treatments witnessed a recurrence of the disease once the medication was stopped.
Dificid inhibits RNA polymerase
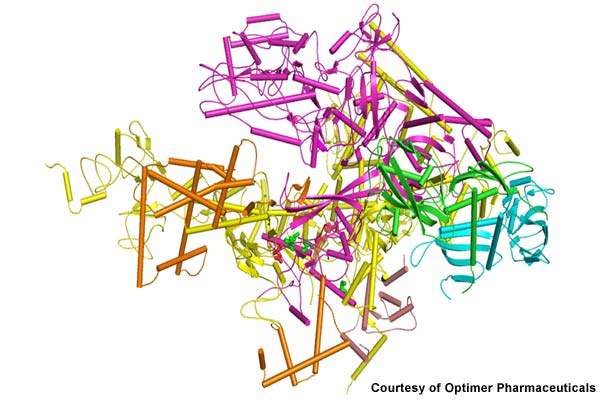
Rather than merely halting bacteria growth, Dificid kills C. difficile by inhibiting RNA polymerase, a bacterial enzyme that kills specific bacteria. Unlike broad-spectrum antibiotics, Dificid is a narrow-spectrum antibiotic that maintains the natural balance of flora while destroying C. difficile in the gastrointestinal tract.
It has minimal systematic exposure, low resistance from C. difficile and a convenient dosing regimen. By returning the colon’s physiological conditions to normal, Dificid-based treatment also results in a lower recurrence rate.
Clinical trials
The FDA approval for Dificid was based on two double-blind, randomised and multicentre studies.
The superiority of Dificid was proved over Vancocin in the first Phase III clinical trial that was performed on over 629 adults. Conducted at over 100 clinical sites across North America, the trial demonstrated that a ten-day course of Dificid is as safe and at least as effective as a same-period course of Vancocin for treating C. difficile infections. Patients were dosed with either 200mg of Fidaxomicin twice daily or 125mg of Vancocin four times in a day in the multi-centre, randomised, double-blind clinical trial.
Results proved that Dificid not only has a higher cure rate globally but also resulted in lower recurrence among patients. Patients required no CDI medication two days after the treatment was completed and showed no recurrence even after four weeks of ending the therapy. The results showed that 88% of the Dificid-administered patients experienced a clinical response, compared to 86% of vancomycin-treated patients.
The second Phase III clinical trial, evaluated the safety and efficacy of Dificid in comparison to Vancocin in 536 adults. The multicentre, randomised, double-blind trial was conducted at approximately 100 sites throughout North America and Europe.
CDI patients were administered with either Dificid 200mg twice a day or oral Vancocin 125mg four times daily for a period of 10 days.
In October 2010, Optimer presented the results of two phase III clinical trials at the 48th Annual Meeting of the Infectious Disease Society of America (IDSA) in Vancouver, Canada. The two Phase III trials on CDI patients with Dificid showed 47% better results compared to vancomycin.
It was also found in the studies that the global cure rates for Dificid patients was 75%, compared to 63% in vancomycin patients. The two trials also indicated that Dificid was safe and well tolerated.
Oral suspension formulation
Apart from the tablet form, Dificid will be developed as an oral suspension formulation by Optimer Pharmaceuticals. The formulation will be used within intensive care units and for elderly patients. Optimer Pharmaceuticals is also planning to file for an Investigational New Drug application to develop Dificid for additional indications.