Fingolimod (branded Gilenya) is an innovative treatment for multiple sclerosis (MS) under development by Novartis. The drug was given fast track designation by the US Food and Drug Administration (FDA) in 2006. It was approved by the FDA in September 2010. With the approval, fingolimod became the first oral medication to be marketed in the US for relapsing forms of MS.
On 24 January 2011, Gilenya was approved by Swissmedic (the Swiss agency for therapeutic products) and the Australian Therapeutic Goods Administration for the treatment of relapsing-remitting MS.
Gilenya was approved by the European Medicines Agency on 21 March 2011 for the same indication.
Improving treatment options for MS patients
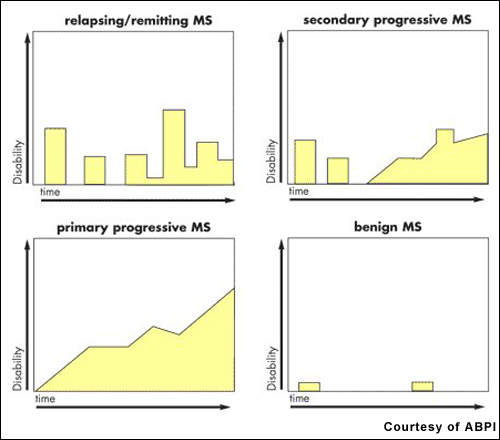
Estimates suggest that about 2.5 million people worldwide have MS. After trauma, it is the second most common neurological disability to affect young and middle-aged adults. It affects twice as many women as men, with the relapsing-remitting forms of MS being the most common.
The advent of the first generation of disease-modifying drugs, including interferon beta-1a, 1b and glatiramer acetate, represented an important advancement in the treatment of MS when introduced into clinical practice.
Approved for the treatment of relapsing forms of MS, they reduce the frequency and severity of exacerbations as well as the number of lesions seen on MRI. However, while these agents have an immunomodulatory effect that alters the course of the disease, they do not reverse the neurological damage that occurs in MS.
There remains a need for more efficacious drugs for MS, especially primary progressive MS, the most aggressive form of the disease. Available disease-modifying agents have to be given parenterally, requiring either daily or weekly injections depending on the medication. Novartis’ fingolimod is a once-daily oral drug with potential disease-modifying effects; as such it would offer a significant benefit over injectable agents.
S1P-R modulators represent a new approach
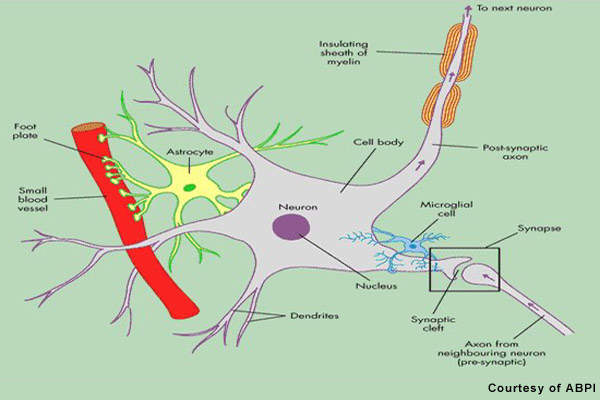
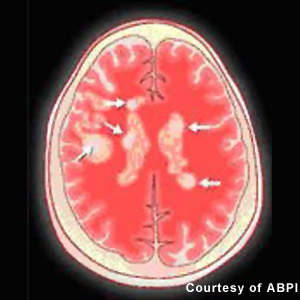
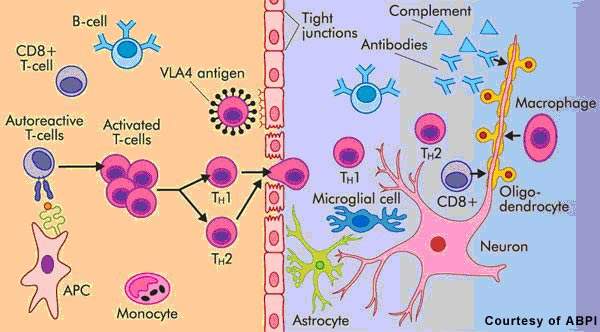
Patients with MS display a range of symptoms that arise from demyelination in the central nervous system (CNS), which includes the brain, spinal cord and optic nerves. The destruction of the protective myelin sheath that surrounds nerve cells is thought to be due to the effects of inflammatory T cells.
Novartis’ fingolimod is a sphingosine 1-phosphate receptor (S1P-R) modulator that binds to the S1P receptor on circulating lymphocytes, sequestering them in lymph nodes away from the CNS. The first oral S1P-R modulator to be developed, it reduces the number of inflammatory T cells in the circulation and CNS and in so doing reduces their potential to damage nerve cells.
Data from preclinical studies suggest that fingolimod may mediate its effects through a variety of mechanisms, some of which may be independent of lymphocyte depletion. For example, imaging studies in an animal model of MS point to enhanced myelination and axonal protection following the oral administration of fingolimod.
Thus, in addition to its anti-inflammatory effects in MS, this novel therapy may have the potential to reduce neurodegeneration as well as promote endogenous repair of the CNS. No marketed treatments for MS can produce remyelination.
Fingolimod Phase III development
Results from the Phase II trial showed that treatment with fingolimod produced sustained reductions in relapses and inflammation in patients with relapsing-remitting MS.
Keeping the safety profile in check, data from the extended use of the Phase II trial confirmed a very low relapse rate in a case sheet submitted to the American Academy of Neurology. Long-term results from the open-label Phase II extension study showed sustained low rates of relapse even after four years of treatment with fingolimod.
On the back of encouraging Phase II results, a global Phase III programme was initiated. It focused on patients with relapsing-remitting MS, included the FREEDOMS (FTY720 Research Evaluating Effects of Daily Oral therapy in Multiple Sclerosis) AND TRANSFORMS (TRial Assessing injectable interfreroN vS FTY720 Oral in RrMS) trials. FREEDOMS, a randomised, double-blind and placebo-controlled trial, assessed the efficacy and safety of fingolimod in 2,000 patients over a 24-month period. Objectives included the impact of treatment on frequency of relapses, disability progression, and lesions as assessed by MRI.
TRANSFORMS, a 1,000+ patient study, compared the efficacy and safety of fingolimod with interferon-beta-1a. Relapse rate at one year was the primary efficacy endpoint in the randomised, double-blind, active-control study.
In TRANSFORMS, 87% of patients who had completed the study on treatment, fingolimod was generally well tolerated with a good success rate. About 10% of patients showed few adverse effects such as headache, nasopharyngitis and fatigue. Other adverse events shown by fingolimod treated patients included fleeting reductions in heart rate at the beginning of treatment, slight increase in blood pressure, increase in liver enzymes and few cases of macular oedema.
Bradycardia and atrioventricular block, malignancies and dyspnea were reported in less than 2% of fingolimod-treated patients, in terms of serious adverse events and infections. About 80–83% patients who were administered with fingolimod were found free of relapses.
Novartis completed its Phase III clinical studies and submitted a new drug application to the FDA and the EMA in December 2009.
Marketing commentary
Multiple sclerosis is a chronic and disabling disease, with healthcare costs disproportionate to the numbers affected. In the US, costs are estimated to exceed $10bn a year. The introduction of interferon beta-1a revolutionised treatment for many patients with MS and fuelled worldwide market growth for MS therapies.
Drug safety has been a concern with some of the newer MS treatments and is something on which regulatory authorities are increasingly focusing their attention. Few safety issues have arisen in Phase II trials with fingolimod. Nonetheless, analysts see fingolimod as one of the most promising oral agents for MS.