NexBio, a US start-up biopharmaceutical company that specialises in developing antiviral agents, has pioneered the development of Fludase (DAS181), an innovative, recombinant drug for the treatment and prevention of influenza.
Fludase completed its initial preclinical development and entered clinical development to determine its efficacy and safety in humans.
In preclinical studies, Fludase displayed potent antiviral activity against clinical influenza isolates, including isolates of the deadly H5N1 strain of avian influenza.
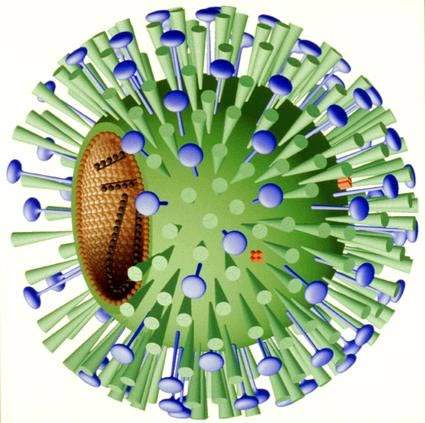
Fludase blocks viral entry into respiratory cells
NexBio’s Fludase is a recombinant sialidase fusion protein composed of a sialidase catalytic domain derived from actinomyces viscosus, a constituent of the normal oral and gastrointestinal flora in humans, and a cell surface-anchoring domain.
In the human respiratory tract, cell surface sialic acids act as host cell receptors for influenza A and B viruses. Fludase works by removing sialic receptors from the airway epithelium, therefore preventing viral entry into cells of the respiratory epithelium.
As influenza viruses primarily invade cells of the upper and central respiratory tract, Fludase is administered through oral inhalation. The cell surface anchoring-domain of Fludase is designed to attach the sialidase to the respiratory epithelium, thereby increasing retention time and drug potency.
Preclinical in vitro and in vivo studies have shown that Fludase possesses potent antiviral and cell protective properties when combined with a long duration of action.
Prophylactic administration of Fludase significantly improved lung function, lung pathology and survival in mice subjected to influenza viral challenge.
In another in vivo model that closely resembles human influenza infection, administration of Fludase led to a significant inhibition of viral replication (viral shedding) accompanied by reduced signs of inflammation and illness. Crucially, there was no evidence that Fludase was toxic to respiratory cells.
Fludase appears effective in cleaving the sialic acid receptors that are used by both human and avian influenza viruses to invade respiratory epithelial cells. It represents a first-in-class influenza therapy and potentially an important new weapon in the fight against new strains of the influenza virus, including new avian strains.
Clinical trials of Fludase
In preclinical studies, Fludase was effective against laboratory and clinical strains of influenza, including the H5N1 strain.
In October 2007, NexBio initiated a Phase I clinical trial of Fludase in healthy adults. The study enrolled 36 adults aged between 18 and 65 years old, and was designed to test the safety of the drug in healthy subjects. The study was completed in January 2009 and revealed that the drug was well tolerated at all dosage levels.
In a separate study conducted at the CDC and published in November 2009, Fludase was found effective against strains of pandemic influenza A (H1N1). Studies carried out at NexBio revealed Fludase was highly active against viruses resistant to Tamiflu.
In January 2010, NexBio initiated a double blind and placebo-controlled Phase II clinical trial of Fludase for treating influenza infection. The study, which will be conducted in 50 centres across the US and Mexico, will measure the effect of the drug on influenza viral load. It will also assess the safety and tolerability of the drug.
NexBio is carrying out Phase II trials using a $6m Phase II SBIR grant provided by the National Institute of Allergy and Infectious Diseases (NIAID), part of the National Institutes of Health.
In July 2010, NexBio started a Phase I clinical trial of Fludase to assess its safety and tolerability in patients suffering from asthma or bronchiectasis. The study enrolled 24 patients and was completed in December 2010.
In May 2010 NexBio initiated a clinical trial to study the effects of the drug in patients with stable and well-controlled pulmonary disease. The study is being conducted in collaboration with the NIAID.
A Phase II study in 50 enrolled patients with parainfluenza started in July 2011. It is expected to be completed in June 2014. In August 2011, the company reported encouraging results of the DAS181 drug in four patients with life-threatening parainfluenza pneumonitis (PIV3).
A severely immunocompromised patient was treated with Fludase, under the Emergency Investigational New Drug Application (eIND) granted by the US FDA, after undergoing an allogeneic stem cell transplantation. It showed improvement in the patient’s respiratory status.
The spectre of avian influenza
Influenza is a highly infectious disease caused by infection with influenza A or B viruses. It is characterised by recurrent annual epidemics and, more rarely, major global pandemics. Research work carried out in the 1960s suggests pandemics usually arise when strains of avian and human influenza combine.
The emergence in the late 1990s of a new avian strain of influenza A, known as H5N1, has caused particular concerns about the possibility of another global pandemic. This highly virulent avian strain has been responsible for widespread infection in poultry flocks in the Far East and sporadic outbreaks in other parts of the world. Wild birds have also been infected. There have been more than 200 documented cases of H5N1 infection in humans, about half of which proved fatal.
In preclinical studies, Fludase was effective against both laboratory and clinical strains of influenza, including the H5N1 strain, so it may have potential to treat this deadly strain.
Expanding treatment options for influenza
Given the huge direct and indirect socioeconomic impact of influenza, finding new ways to combat this potentially life-threatening disease is a clinical priority.
Vaccination is an established means of disease prevention, while antiviral agents are primarily indicated for treatment.
Neuraminidase inhibitors, such as oseltamivir and zanamivir, are the major class of antiviral drugs used to treat influenza. They selectively inhibit viral neuramindase, a surface enzyme which is critical for influenza viral replication in the respiratory tract.
These drugs are effective in reducing the duration of illness and risk of complications.
However, their efficacy diminishes significantly if they are not taken within 48 hours of the onset of symptoms. Influenza strains can also develop resistance to these drugs, reducing their clinical effectiveness.
Fludase differs from licensed antiviral drugs – such as the neuramindase inhibitors – in that it targets host respiratory cells rather than the influenza virus itself.
It therefore represents an entirely new approach to combating influenza, with the potential to treat all influenza strains, including strains resistant to other antiviral drugs. Potentially, it could also be used for the treatment of other significant respiratory viruses.
Marketing commentary for NexBio’s experimental drug Fludase
Fludase is one of three drugs being developed by NexBio for the treatment of influenza. The others are Inviridin (NEX-PIN) and Viracidin (NEX-VAC). NexBio hopes these antiviral agents will address some of the problems associated with current vaccines and antiviral drugs, as well as expanding treatment options for influenza.
The existing market for antiviral drugs for influenza already exceeds $2bn a year. Analysts believe this will more than double if patient-needs for effective influenza prophylaxis and treatment could be met in full.