Hizentra® (immune globulin subcutaneous 20% liquid) is a subcutaneous infusion indicated for the treatment of patients with chronic inflammatory demyelinating polyneuropathy (CIDP).
Discovered and developed by CSL Behring, Hizentra® is one of the first subcutaneous immunoglobulins to be approved for the treatment of CIDP.
CSL Behring’s biologics license application (BLA) for Hizentra® was accepted for review by the US Food and Drug Administration (FDA) in July 2017 and approved in March 2018.
The drug also received marketing authorisation (MA) approval in Europe in January 2018.
Chronic inflammatory demyelinating polyneuropathy causes and symptoms
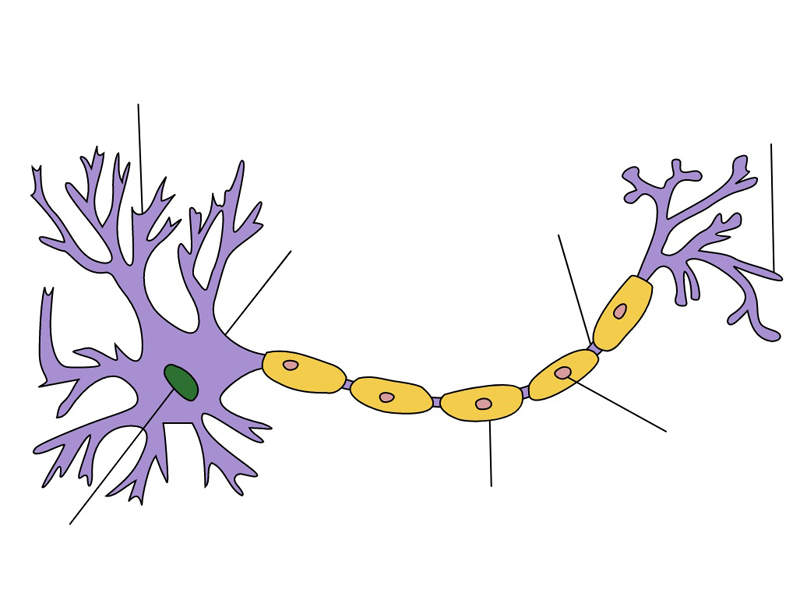
CIDP is a neurological and autoimmune disorder that affects the peripheral nerves. The disorder leads to impaired sensory function in the legs and arms.
The disease is mainly caused by the damage on the protective covering of the nerves, which may cause numbness or tingling, fatigue and muscular weakness. It is also known as chronic relapsing polyneuropathy when it causes damage to the myelin sheath of the peripheral nerves.
An estimated 30% of CIDP patients become wheelchair dependent if not treated. The incidence of CIDP is reported in up to two out of 100,000 people in the US.
Hizentra’s mechanism of action
Hizentra® contains human normal immunoglobulin, which is a highly purified protein derived from human plasma.
The drug produces a range of immunomodulatory effects by supplying opsonising and neutralising Immunoglobulin G (IgG) antibodies that work against bacterial and viral agents to prevent relapse of neuro-muscular disability and impairment.
Phase III clinical trials on Hizentra
The FDA’s approval for Hizentra® was based on data from the Phase III polyneuropathy and treatment with Hizentra® (PATH) study. This multi-centre, double-blind, randomised, placebo-controlled, parallel-group clinical study was conducted to evaluate the safety and efficacy of Hizentra®.
The study enrolled 172 adult patients with CIDP that were previously treated with immune globulin intravenous (IGIV). The patients received either weekly doses of Hizentra® in 0.4g per kilogram body weight and 0.2g per kilogram body weight doses, or placebo.
The average treatment duration was 129 days for the patients taking Hizentra® at a dose of 0.4g/kg, while it was 118.9 days in the 0.2g/kg Hizentra® group.
The primary endpoint of the percentage of patients who experienced a CIDP relapse or withdrawn for any other reason during subcutaneous immunoglobulin (SCIg) treatment was significantly lower in the Hizentra® group when compared to the placebo group.
The most common adverse reactions found in the clinical studies were headaches, diarrhoea, fatigue, back pain, nausea, extremity pain, coughing, upper respiratory tract infection, rash, pruritus, vomiting and migraine.
Marketing commentary on CSL Behring
CSL Behring is a subsidiary of CSL, a biopharmaceutical company based in Australia.
CSL operates in more than 60 countries, employing more than 20,000 people. It manufactures therapeutic products for the treatment of bleeding disorders, immune deficiencies, neurological disorders and alpha-1 antitrypsin deficiency.
These products are used in cardiac surgery, organ transplantation, and prevention of haemolytic disease in newborns.