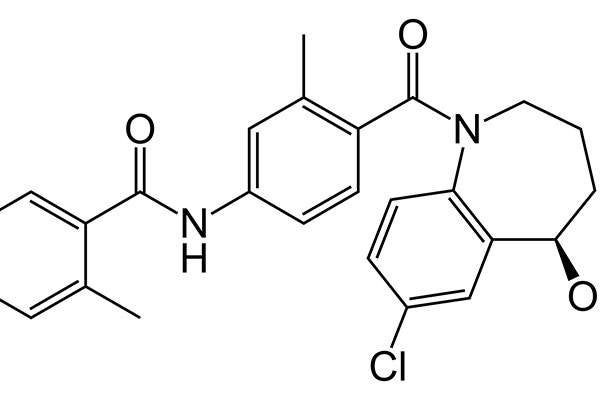
JYNARQUE™ (tolvaptan) is indicated for the treatment of autosomal dominant polycystic kidney disease (ADPKD).
The drug was discovered and developed by Otsuka Canada Pharmaceutical.
Otsuka received Health Canada’s approval for JYNARQUE™ in February 2015, and it was granted orphan drug designation by the European Commission (EC) in August 2013. It was also recommended for marketing authorisation by the European Medicines Agency (EMA) in February 2015, which was approved in May 2015.
The US Food and Drug Administration (FDA) approved JYNARQUE™ in April 2018, and it was also approved in Japan sold under the brand name Samsca.
In addition, the drug was approved in South Korea, Australia, Switzerland, Hong Kong, Turkey and Taiwan.
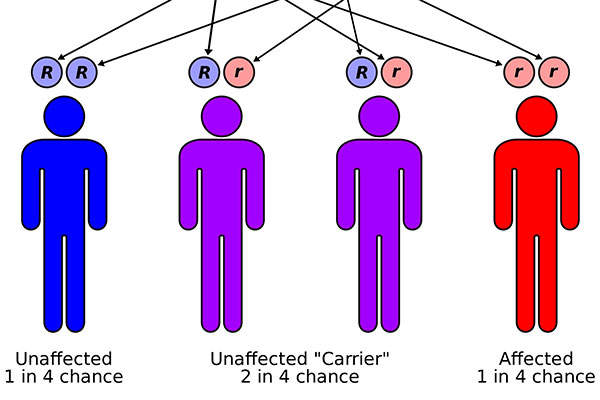
Autosomal dominant polycystic kidney disease
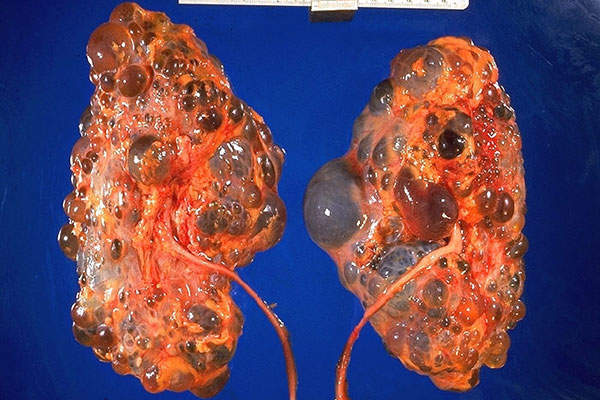
ADPKD is a genetic disease characterised by the development of multiple, non-malignant cysts in the kidneys. It leads to complications, including chronic and acute pain, hypertension and kidney failure.
It is estimated that ADPKD affects 35,000 people in Canada and 205,000 people across Europe.
Jinarc’s mechanism of action
JYNARQUE™ contains vasopressin-2-receptor antagonist, which inhibits the vasopressin-2-receptors in the kidneys and regulates the level of water and sodium in the body. The drug also reduces the development and growth of kidney cysts.
The drug is available in 15mg, 30mg, 45mg, 60mg and 90mg tablets for oral administration.
Clinical trials on Jinarc
Omontys (peginesatide) is an erythropoiesis-stimulating agent (ESA) developed for the treatment of anaemia associated with chronic kidney disease (CKD).
Clinical development of JYNARQUE™ was undertaken by Otsuka in collaboration with the world’s leading ADPKD medical specialists in 2004 at its Japanese pharmaceutical research centre.
Health Canada and the US FDA’s approvals, as well as the EMA’s positive recommendation for approval in Europe were based on results from a Phase III clinical study called TEMPO 3:4 trials.
The study was a randomised, double-blind and placebo-controlled trial conducted from January 2007 to January 2009. It enrolled more than 1,445 adult patients with early and rapidly progressing ADPKD.
The results demonstrated that patients treated with JYNARQUE™ achieved the primary endpoint of demonstrating a statistically significant reduction ( 49%) of the annual increase in total kidney volume (TKV). JYNARQUE™ was also found to reduce the decline in kidney function by 30%.
The most commonly reported adverse reactions in the JYNARQUE™ administered group included thirst, polyuria, nocturia and pollakiuria.
Marketing commentary
Otsuka Canada Pharmaceutical is a subsidiary of Otsuka Pharmaceutical. Established in 2010 in Canada, it is a fast-growing healthcare company that is headquartered at Saint-Laurent, Québec.
The company commercialises Otsuka’s medicines in Canada, primarily focusing on neuroscience, nephrology, oncology and cardiovascular drugs.