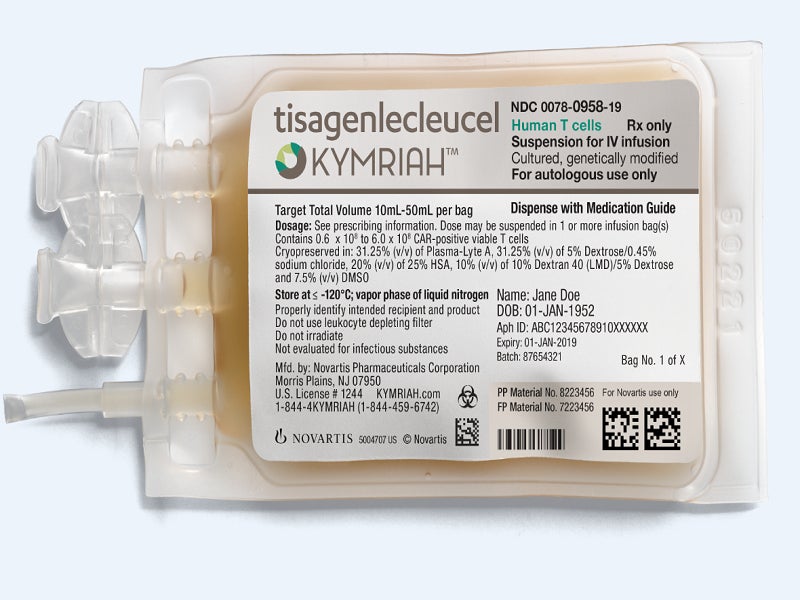
Kymriah™ (tisagenlecleucel) is a chimeric antigen receptor T-cell (CAR-T) therapy that is approved in the US for the treatment of paediatric and young adult patients with relapsed or refractory (r/r) B-cell precursor acute lymphoblastic leukaemia (ALL), as well as adults with r/r large B-cell lymphoma and r/r follicular lymphoma (FL).
The drug was first developed at the University of Pennsylvania using a 4-1BB costimulatory domain for enhancing cellular responses. Swiss-American pharmaceutical company Novartis subsequently joined as the drug’s co-developer as part of a development contract.
Regulatory approvals of Kymriah
Kymriah received Priority Medicines (PRIME) designation from the European Medicines Agency (EMA) in 2016.
In March 2017, Novartis’ biologics license application (BLA) for Kymriah was accepted for review by the US Food and Drug Administration (FDA). The FDA granted breakthrough therapy designation to the drug in April 2017.
In July 2017, the FDA’s Oncologic Drugs Advisory Committee (ODAC) unanimously recommended Kymriah’s approval. The drug subsequently received full FDA approval in August 2017.
In May 2018, the FDA approved the drug to treat adult patients with relapsed or refractory (r/r) large B-cell lymphoma, including diffuse large B-cell lymphoma (DLBCL), high-grade B-cell lymphoma, and DLBCL occurring from follicular lymphoma after two or more lines of systemic therapy.
In August 2018, the European Commission (EC) approved Kymriah for the treatment of B-cell ALL in paediatric and young adult patients, as well as for adults with r/r DLBCL.
In April 2020, Kymriah received regenerative medicine advanced therapy (RMAT) designation from the FDA for r/r FL.
In May 2022, the drug was granted approval for r/r FL by the FDA and the EC based on results from the Phase II ELARA trial.
Acute lymphoblastic leukaemia details
ALL is a type of cancer that occurs due to the uncontrolled growth of white blood cells, which crowd other types of cells in the bone marrow and prevent the production of red blood cells and platelets.
The disease causes patients to become anaemic and prone to infections, bleeding and bruising.
ALL is estimated to account for 25% of cancer diagnoses among children younger than 15 years in the US. The risk of developing ALL is highest in children younger than five years.
B-cell lymphoma is a group of cancers that grow in the B-lymphocytes, a part of the immune system, and attacks them. FL is a slow-growing or indolent form of non-Hodgkin lymphoma that originates from B-lymphocytes, making it a B-cell lymphoma.
Kymriah’s mechanism of action
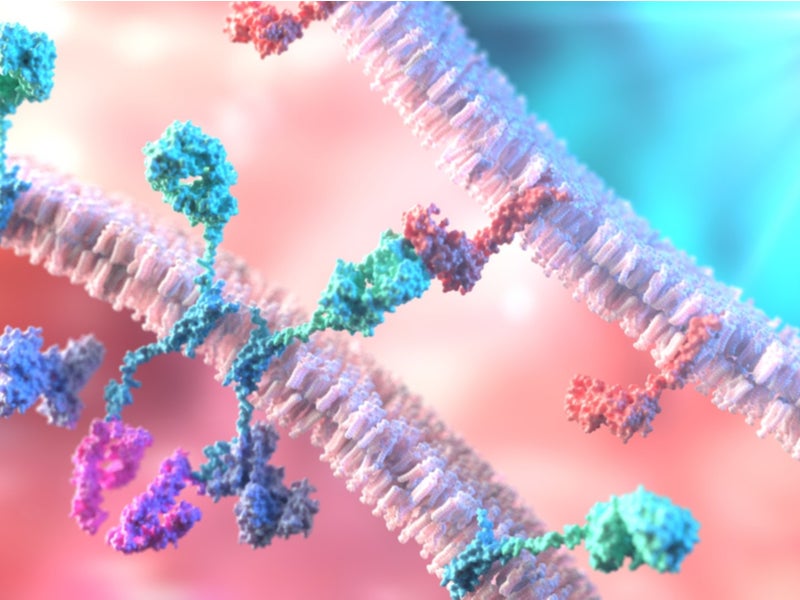
Kymriah is a chimeric antigen receptor T-cell (CAR-T) therapy that reprograms the patient’s T-cells with a transgene encoding a chimeric antigen receptor (CAR) while identifying and removing CD19-expressing malicious cells.
The drug is available in 10ml and 50ml suspensions for each bag and can be administered through intravenous (IV) infusion.
Clinical trials on Kymriah
The FDA’s approval of Kymriah for B-cell ALL was based on results from a pivotal open-label, multi-centre, single-arm Phase II clinical trial named ELIANA.
The trial was conducted across 25 centres in the US, EU, Canada, Australia, and Japan. It involved a total of 88 patients being infused with Kymriah.
The study demonstrated that 83% of the patients treated with Kymriah achieved complete remission with incomplete blood count recovery within three months of infusion.
Results also indicated that 63 patients treated with Kymriah demonstrated relapse-free survival at six months and had no minimal residual disease, which is an indicator for potential relapse detected among responding patients.
The most common adverse reactions encountered during the clinical study were cytokine release syndrome (CRS), hypogammaglobulinaemia, unspecified pathogen infections, pyrexia, decreased appetite, headache, encephalopathy, hypotension, and bleeding episodes.
Other adverse reactions reported in the trial included tachycardia, nausea, diarrhoea, vomiting, viral infectious disorders, hypoxia, fatigue, acute kidney injury, and delirium.
The FDA’s approval of Kymriah for r/r DLBCL in adult patients was based on positive results from a Phase II clinical trial named JULIET, which enrolled a total of 106 patients. Out of 68 Kymriah-infused patients evaluated for efficacy, the overall response rate was 50%, with 32% of patients showing complete response and 18% achieving partial response.
Kymriah was approved for r/r FL based on a multi-centre, single-arm, open-label Phase II clinical trial named ELARA. In the trial, Kymriah showed high response rates in pre-treated patients, including an 86% overall response rate and a 69% complete response. The drug’s safety profile was also found to be ‘remarkable’.
In addition, Kymriah demonstrated a prolonged durable response to treatment in patients, with around 87% of the patients who had achieved complete response still in response at or more than nine months after initial response.
The most common adverse events reported in patients during the study were CRS, infections, high body temperature, diarrhoea, nausea, fatigue, low blood pressure, oedema, and headaches.
Marketing commentary on Novartis
Novartis is a healthcare solution provider based in Basel, Switzerland. The company offers innovative medicines, generic and biosimilar pharmaceuticals, and eye care. Its products are sold in more than 155 countries.
Novartis and the University of Pennsylvania originally entered a development contract in 2012, which led to the two companies agreeing to carry out the development and commercialisation of CAR-T cell therapies, including Kymriah.
The commercial manufacturing of Kymriah is carried out at Novartis’ manufacturing facility in Morris Plains, New Jersey.