Lynparza (olaparib), discovered and developed by AstraZeneca, is the first PARP inhibitor drug approved for germline BRCA-mutated advanced ovarian cancer.
The US Food and Drug Administration (FDA), in December 2014, approved Lynparza (olaparib) twice daily capsule as the first monotherapy for patients with deleterious or suspected deleterious germline BRCA-mutated (gBRCAm) advanced ovarian cancer, who already received three or more prior lines of chemotherapy treatment.
The drug received accelerated approval from the FDA based on results from Phase II clinical trials. Phase III trials are still going on, and further approval will be based upon verification of clinical benefits.
AstraZeneca submitted an amended new drug application (NDA) for the drug in July 2014 after receiving a request from the FDA for additional data. The first NDA was submitted in February 2014.
The European Commission (EC) also granted marketing authorisation for Lynparza in December 2014 as the first therapy for the maintenance treatment for adult patients with platinum-sensitive relapsed BRCA-mutated (germline and / or somatic) high-grade serous epithelial ovarian, fallopian tube, or primary peritoneal cancer, who are in complete or partial response to platinum-based chemotherapy.
The drug will be available in all 28 EU member states, as well as Norway, Iceland and Liechtenstein.
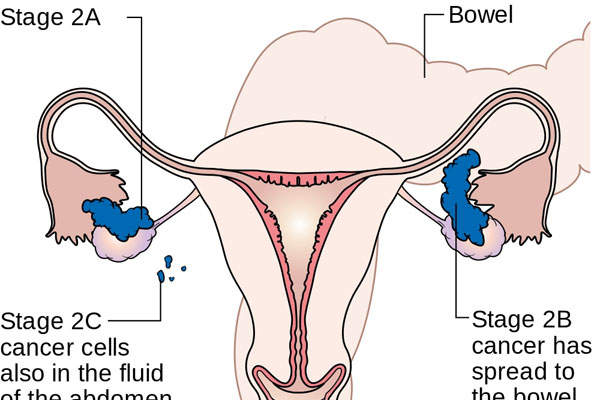
Ovarian cancer and BRCA-mutated ovarian cancer
Ovarian cancer affects the ovaries in the female reproductive system. It is the fifth leading cause of cancer-related death among women in the US. In many cases, the survival rate is low because the cancer is often diagnosed late.
BRCA mutation is a mutation in the either BRCA1 genes or BRCA2 genes. These mutations disable an important DNA repair process, and significantly increase the risk of developing ovarian cancer and other cancers. BRCA mutation affects up to 15% of ovarian cancer patients.
Lynparza’s mechanism of action
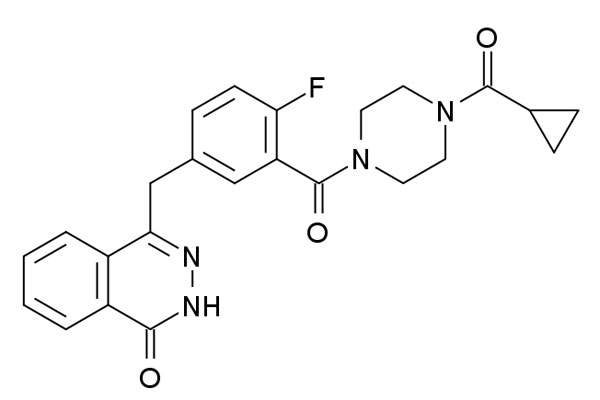
Lynparza contains an active component called olaparib, which is a poly ADP ribose polymerase (PARP) inhibitor. It exploits tumour DNA repair pathway deficiencies and kills cancer cells. This mechanism of action gives the drug the potential to fight against a range of tumours with DNA repair deficiencies.
Lynparza will be available in the form of 400mg capsules for oral administration only.
Clinical trials on Lynparza
Kadcyla (trastuzumab emtansine) is an antibody drug conjugate indicated for the treatment of HER2-positive metastatic breast cancer (mBC).
The FDA approval for Lynparza is based on results from Phase II clinical trials conducted on patients with deleterious or suspected deleterious germline BRCA-mutated advanced cancers to determine safety and efficacy of the drug.
The Phase II clinical trial, which aimed to determine the efficacy of Lynparza, was a single-arm, open-label study that enrolled 137 gBRCA-mutated ovarian cancer patients who received three or more prior lines of chemotherapy.
The study met all of its primary end points with a response rate of 34%. The median response duration was 7.9 months. The common adverse reactions recorded were nausea, vomiting, anaemia and fatigue.
A Phase III clinical trial known as SOLO is in progress. Results from the studies are crucial for full approval of the drug, which currently holds accelerated approval. The SOLO programme, which was initiated in September 2013, includes three individual Phase III clinical trials known as SOLO1, SOLO2 and SOLO3.
SOLO1 evaluates olaparib as a maintenance monotherapy for ovarian cancer patients who were completely or partially responsive to first-line platinum-based chemotherapy.
SOLO2 evaluates olaparib as a maintenance monotherapy for relapsed ovarian cancer patients who were completely or partially responsive to first-line platinum-based chemotherapy.
SOLO3 compares olaparib with non-platinum chemotherapy as third-line or later treatment for relapsed ovarian cancer patients.
AstraZeneca is also investigating the potential of olaparib in multiple tumour types. It is simultaneously conducting Phase III studies in adjuvant and metastatic BRCA-mutated breast cancers, BRCA-mutated pancreatic cancer and second line gastric cancer.