MOR103 is a fully human antibody indicated for the treatment of rheumatoid arthritis (RA). The drug is being developed by Germany-based MorphoSys in collaboration with Australia’s University of Melbourne. MOR103 is produced using MorphoSys’s advanced technology called Human Combinatorial Antibody Library (HuCAL) PLATINUM.
HuCALs are collections of a range of fully human synthetic antibodies with strong binding characteristics. MorphoSys’s HuCAL PLATINUM technology provides a direct source of these antibodies for use in research and therapeutics.
Under an agreement with MorphoSys, the University of Melbourne is carrying out research to develop MOR103 for a range of indications. The university is also eligible for research funding, clinical milestone and royalty payments.
Rheumatoid arthritis
RA is a chronic inflammatory disorder that affects the body’s joints. It also affects the extra-articular tissues of the skin, heart and muscles. It is estimated that between four and six million people suffer from the disease.
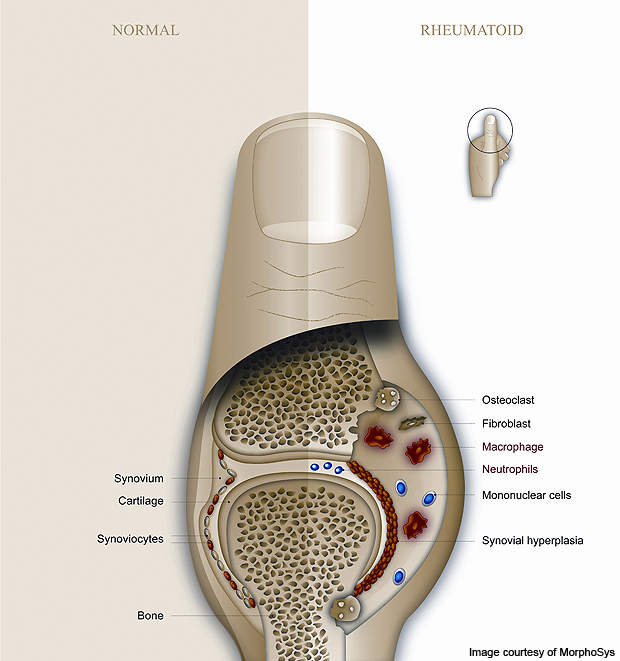
The disease is caused by a disorder in the immune system wherein the body is unable to distinguish between healthy cells and foreign bodies such as viruses and bacteria. It leads to the inflammation of a membrane called the synovium which surrounds each movable joint in the body, creating a protective fluid-filled covering.
With RA, there is an increased flow of white blood cells into the synovium leading to inflammation. The increased flow is produced when the body mistakes the healthy cells for foreign bodies and attempts to fight them by increasing blood flow to the affected area.
In a healthy person, increased blood flow causes inflammation in the affected area. However, in a person suffering from RA, the inflammation leads to acute swelling and the destruction of cartilage and bone tissue. Continuous inflammation can lead to progressive deformation of the joint.
MOR103 – Human HuCAL Antibody
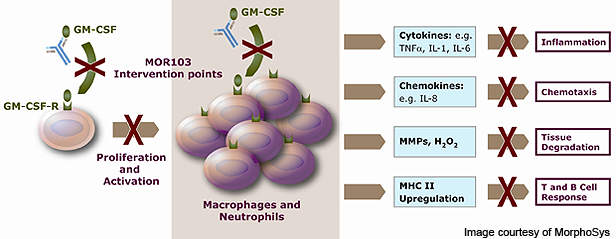
MOR103 works by blocking the action of granulocyte macrophage colony-stimulating factor (GM-CSF), a growth factor for white blood cells. GM-CSF is part of the immune system and is found in high levels in inflamed joints. It is responsible for the increased inflammation caused in immune disorders such as RA.
GM-CSF also plays a major role in the release of signalling molecules such as granulocytes (a category of white blood cell) and other macrophages (white blood cells within tissues).
These molecules activate the B and T cells in the body and also increase the production of pro-inflammatory and pathogenic molecules.
By neutralising GM-CSF, MOR103 prevents the production of granulocytes and other macrophages and their subsequent actions. It also blocks several pathophysiological pathways in the body and disrupts the inflammatory cycle. The mechanism of action of MOR103 is expected to provide avenues for treating a range of inflammatory diseases including RA, multiple sclerosis and asthma.
Clinical trials
In pre-clinical studies, MOR103 showed positive results in rats affected with RA. In December 2007, MorphoSys submitted a clinical trial application to the regulatory authorities in the Netherlands to commence Phase I clinical trials of the drug. The application was approved in February 2008.
The Phase I trial was a randomised, double-blind, placebo-controlled single ascending dose study. It was conducted in 63 healthy volunteers and demonstrated that MOR103 was well tolerated at various dose levels.
In June 2009, MorphoSys presented an application for initiating a Phase 1b/2a clinical trial in patients suffering from RA. The application was approved in November 2009 and the first patient was enrolled in January 2010. MorphoSys is expected to recruit about 135 patients for the trial. Patient enrolment for the trial is expected to be completed in the first half of 2011. Final results from the trial are expected in the first half of 2012.
MorphoSys’s collaboration with the University of Melbourne provides it with exclusive rights to use the inhibitors of GM-CSF through a US patent. In July 2009, the company filed additional patents to investigate MOR103 in new therapeutic areas. The patents will provide MorphoSys with market exclusivity for treating inflammatory diseases using the anti-GM-CSF approach.