Rituxan (Rituximab) is a monoclonal antibody indicated for treating advanced follicular lymphoma. The drug was developed by Biogen Idec and is co-promoted by Genentech, a subsidiary of Roche Group.
Rituxan was approved by the US Food and Drug Administration (FDA) in 1997 for treating non-Hodgkin’s lymphoma. The drug received EU approval in June 1998. It is being sold under the trade name MabThera.
On 28 January 2011, the US FDA approved Rituxan as a maintenance treatment for advanced follicular lymphoma patients who responded to initial treatment with Rituxan plus chemotherapy. The European Commission approved the drug for similar indication in October 2010.
Rituxan is marketed in the US by Genentech and Biogen. In Japan it is marketed by Chugai and Zenyaku Kogyo. Roche markets the drug in the rest of the world. In Roche’s pipeline, Rituxan is the second-largest selling drug after the anti-cancer drug Avastin.
Advanced follicular lymphoma
Follicular lymphoma (FL) is a low-grade and the second most common type of non-Hodgkin lymphoma (NHL). NHL is a type of cancer of the white blood cells. Known as lymphocytes, the disease mainly affects the lymph nodes and can also involve the bone marrow, spleen, waldeyer’s ring and peripheral blood. The median survival of the disease-affected person is eight to ten years.
It is estimated that follicular lymphoma is found in every one out of 3,000 people worldwide. The disease affects about 286,000 people worldwide every year. In 2010, the National Cancer Institute (NCI) estimated that 65,540 new cases of the disease were reported and 20,210 people died in the US.
Targeting the disease
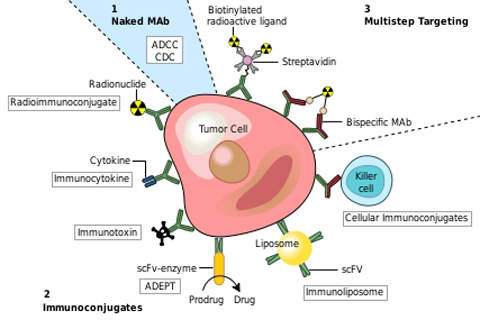
As a therapeutic antibody, Rituxuan targets follicular lymphoma by binding to the antigen CD20 which is present on the surface of the normal or cancerous B-cells. The drug assists the body’s immune system to get rid of the marked CD20 positive B-cells, regenerate new healthy cells from the lymphoid stem cells and return them to normal levels within 12 months.
Rituxan clinical trials
The FDA approval of Rituxan was based on the PRIMA (primary Rituxan and maintenance) Phase III clinical trials. The trials were conducted between December 2004 and May 2007. The study enrolled 1,217 follicular lymphoma patients. It was an open label, multicentre and randomised study with two treatment phases.
The study evaluated the safety and efficacy of Rituxan combined with chemotherapy in treating follicular lymphoma patients. The primary outcome measure was to find the progression free survival (PFS) time from randomisation to progression, relapse or death. The secondary outcome measures included evaluation of response rates, event driven survival endpoints and chemotherapy regimens combined both with and without Rituxan.
Eight cycles of Rituxan combined with different chemotherapy were used as initial treatment. Patients who responded to the initial treatment were randomised to receive Rituxan once every two months, for a period of two years, as a single agent.
The results demonstrated that administration of Rituxan in combination with chemotherapy for the specified period doubled the PFS in follicular lymphoma patients. The study also proved the safety and efficacy of 375mg/m² Rituxan as it was consistent in the previously used pivotal studies when used alone or in combination with chemotherapy than those who did not continue to receive Rituxan.
The patients who received Rituxan reported infections of grade 2. Low white blood cell count and infections were the grade 3-4 adverse reactions which were found higher in Rituxan group.
In December 2010, Roche reported positive results from multicentre, randomised Phase III clinical trials on Rituxan conducted on 462 patients without symptoms of follicular lymphoma.
The study was designed to find the safety and efficacy of Rituxan in three phases. In phase A the patients were not administered with any therapy, but were just observed carefully. In phase B the patients were administered with 375mg/m² of Rituxan weekly for four cycles.
In phase C for four cycles 375mg/m² of Rituxan was administered to the patients followed by Rituxan maintenance, which was given once in every two months.
The results of the study demonstrated that immediate use of Rituxan decreased the risk of necessity for additional therapy by 80% and the risk of disease worsening was reduced by 79% compared with watchful waiting.