Truvada (emtricitabine and tenofovir disoproxil fumarate) is an antiretroviral indicated for preventing or reducing the risk of acquiring HIV-1. The drug is developed and manufactured by Gilead Sciences.
Gilead Sciences received approval for truvada for HIV prevention from the US Food and Drug Administration (FDA), in July 2012.
Details of human immunodeficiency virus
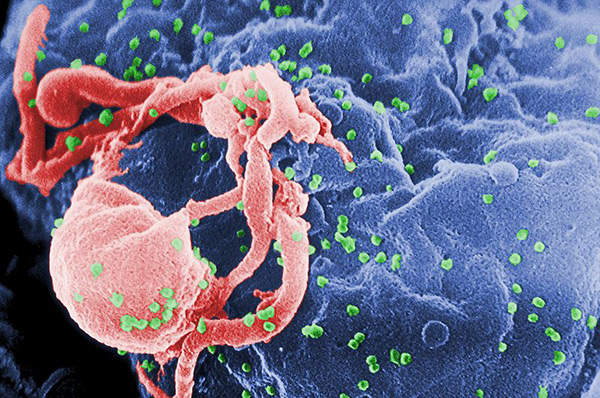
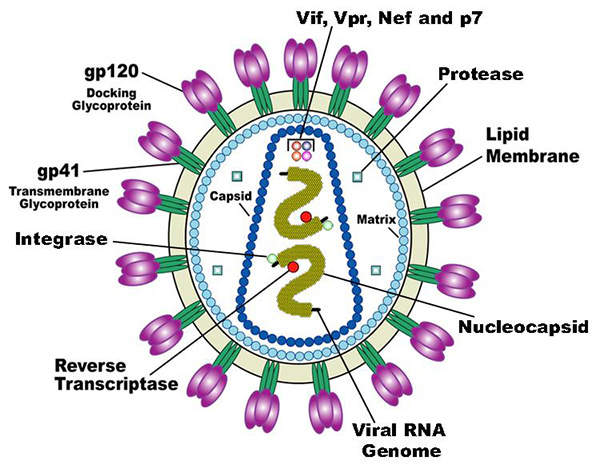
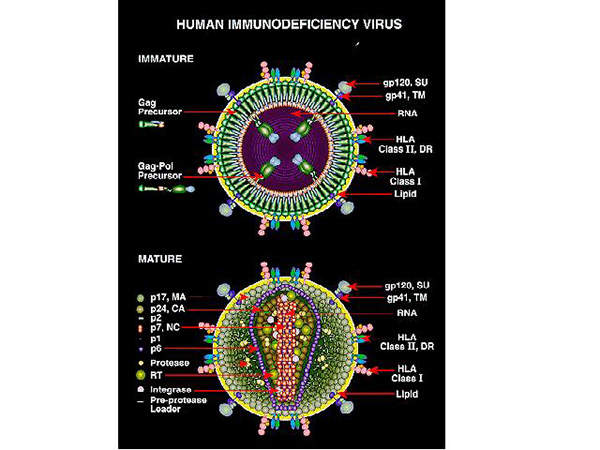
Human immunodeficiency virus (HIV) is a lentivirus, which can deliver a significant amount of genetic information into the DNA of the host cell.
It causes acquired immunodeficiency syndrome (AIDS), a life-threatening disease which weakens the immune system and makes people much more susceptible to infections and diseases. HIV is categorised into two types, HIV-1 and HIV-2. HIV-1 is more virulent and infective than HIV-2.
It is currently estimated that about 1.2 million people in the US are living with HIV and more than 50,000 new cases of infections are being registered each year. It is also found that about 23% of the new HIV cases are found in women, while in men it is 61%.
How truvada helps prevent HIV
Truvada is made with a combination of two nucleoside reverse transcriptase inhibitors (NRTI) drugs, which include emtricitabine and tenofovir disoproxil fumarate.
Emtricitabine restrains reverse transcriptase, an enzyme that copies HIV RNA into new viral DNA. It also lowers the amount of HIV and increases the immune system cells in the patient’s body. Tenofovir disoproxil fumarate also blocks the reverse transcriptase.
Clinical trials on the antiretroviral drug
FDA approval for truvada was based on a Phase III clinical trial, known as the Pre-Exposure Prophylaxis Initiative (iPrEx). It was a randomised, double-blind and parallel assignment conducted on 2,499 HIV uninfected males.
It was sponsored by the US National Institutes of Health (NIH) and the Bill and Melinda Gates Foundation.
The primary outcome measure of the study was finding anti-HIV seroconversion and safety endpoints in 36 months. The secondary outcome measures included finding changes in bone mineral density, drug resistance in HIV patients and prevalence of sexually transmitted infections in 42 months.
The results of the iPrEx study showed that truvada reduced the risk of acquiring HIV infection by 42%. The study also found that truvada reduced infection rates by 43.8% in homosexual men.
Related project
Eviplera – Treatment for HIV, United States of America
Eviplera is a single-pill regimen targeted at human immunodeficiency virus-1 (HIV-1).
Another Phase III clinical trial on the drug was also considered for the FDA approval. It is known as partners PrEP trial which enrolled 4,758 heterosexual couples, where one partner was HIV-infected and the other was not.
It was sponsored by University of Washington and Bill and Melinda Gates Foundation. It was conducted between May 2008 and June 2012. It was a randomised, double-blind and parallel assignment. The trial evaluated the safety and efficacy of truvada in comparison with a placebo.
The results of the partners PrEP trial showed that truvada reduced the risk of becoming HIV-infected by 75% when compared to the placebo. The general side effects of the drug included pain, weight loss and diarrhoea.
Gilead conducted Phase IV clinical trials on truvada between July 2008 and April 2011. It was an open label, randomised, multicentre and parallel assignment. It evaluated the safety and efficacy of the drug in the treatment of HIV-1 infected subjects.
The primary outcome measure of the study was finding the percentage of participants with HIV-1 Ribonucleic Acid after 48 weeks. The secondary outcome measures included finding the percentage of people with Pure Virologic Response (PVR) and change from baseline in cluster determinant 4 after 48 weeks.
Marketing commentary on Gilead’s HIV prevention drugs
Gilead has commercially launched truvada in the US market. The company faces competition from other medications available in the US market for the same indication, which include Gilead’s own drugs Viread (tenofovir) and Emtriva (emtricitabine), Crixivan (indinavir) manufactured by Merck, Edurant (rilpivirine) manufactured by Johnson & Johnson and Tibotec.