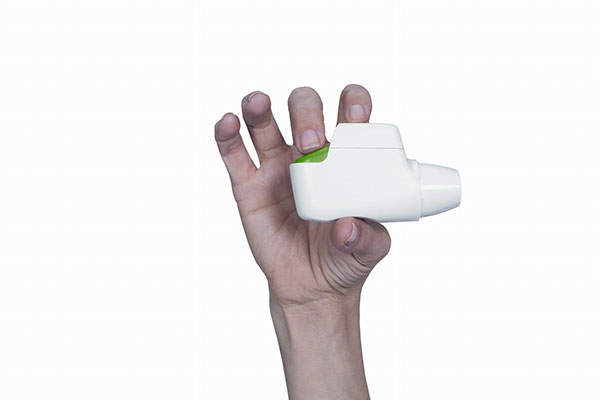
Tudorza Pressair (aclidinium bromide inhalation powder) is an anticholinergic drug indicated for the long-term maintenance treatment of bronchospasm associated with chronic obstructive pulmonary disease (COPD), including chronic bronchitis and emphysema. It was developed by Forest Laboratories in collaboration with Spanish company Almirall.
In July 2012, Forest Laboratories received approval for Tudorza Pressair from the US Food and Drug Administration (FDA).
Chronic obstructive pulmonary disease (COPD)
Chronic obstructive pulmonary disease (COPD) is a lung disease that limits the flow of air to and from the lungs causing shortness of breath. The disease has two forms, namely chronic bronchitis, which involves a long-term cough with mucus, and emphysema, which involves destruction of the lungs. Most of the patients affected by COPD have a blend of both forms of the disease. The main cause of the disease is tobacco smoking.
According to the World Health Organization (WHO), about 64 million people across the world are affected by COPD. It is also estimated that about three million people worldwide died due to the disease in 2005. The disease is predicted to become the third leading cause for death worldwide by 2030. COPD is the third leading cause of death in the US.
Tudorza Pressair mechanism of action
Tudorza Pressair is an anticholinergic drug that exhibits bronchodilatory effect by blocking the neurotransmitter acetylcholine from binding to M3 receptors in the airway smooth muscle.
Tudorza is administered through the Pressair inhaler containing a dry powder formulation of aclidinium bromide which is used for oral inhalation. It is administered twice in a day and delivers 400mcg of aclidinium bromide per actuation.
Aclidinium bromide clinical trials
The FDA approval for Tudorza Pressair was based on a dose-ranging trial (trial A) for nominal dose selection and three confirmatory phase III trials (trials B, C and D).
Trial A was a randomised, double-blind, placebo-controlled, active-controlled, cross-over trial including Tudorza Pressair doses of 400mcg, 200mcg and 100mcg twice daily, formoteroal active control, and placebo. The study’s results showed that the forced expiratory volume in one second (FEV1) in patients treated with Tudorza Pressair 100mcg and 200mcg twice daily doses was lower, when compared to the patients treated with Tudorza Pressair 400mcg twice daily dose.
Trials B, C and D were three randomised, double-blind, placebo-controlled trials evaluating Tudorza Pressair 400mcg twice daily in patients with COPD. Trials B and C were conducted for three months and Trial D had duration of six months. The studies enrolled over 1,276 patients, who were above 40 years of age, had a history of smoking at least 10 pack-years, a forced expiratory volume in one second (FEV1) of at least 30% and less than 80% of predicted normal value, and a ratio of FEV1 over forced vital capacity (FEV1/FVC) of less than 0.7.
The results of the three studies demonstrated that the patients treated with Tudorza Pressair 400mcg twice daily had statistically significant greater bronchodilation when compared to placebo, as measured by a change from baseline in morning pre-dose FEV1 at 12 weeks.
Additionally, serial spirometric evaluations of FEV1 were performed over 12 hours in a subset of patients in the three trials. Improvement of lung function with Tudorza Pressair versus placebo was demonstrated on day 1 and was consistent over the 3 or 6-month treatment period evaluated.
The most common adverse events reported in the clinical trials on Tudorza Pressair included headache, nasopharyngitis and cough.
Marketing Tudorza Pressair
Related project
Spiriva – Treatment for Chronic Obstructive Pulmonary Disease, United States of America
Spiriva is an anticholinergic drug targeted at chronic obstructive pulmonary disease (COPD). It contains tiotropium bromide as the active ingredient and is administered as an oral medication, through the unique HandiHaler inhalation device.
In 2005, Forest Laboratories licensed the marketing rights to Tudorza Pressair in the US from Almirall, while Kyorin Pharmaceutical holds the rights in Japan, and Daewoong Pharmaceutical in Korea. Almirall and Menarin hold joint commercialisation rights in major European member states and other non-EU countries. Almirall holds the rights for the rest of the world.
Other approved COPD medications include Symbicort (budesonide/formoterol fumarate dihydrate) developed by AstraZeneca, Advair (fluticasone propionate) manufactured by GlaxoSmithKline, Spiriva (tiotropium bromide) developed by Boehringer Ingelheim and Pfizer, Daliresp (roflumilast) developed by Nycomed, and Onbrez (indacaterol) developed by Novartis.