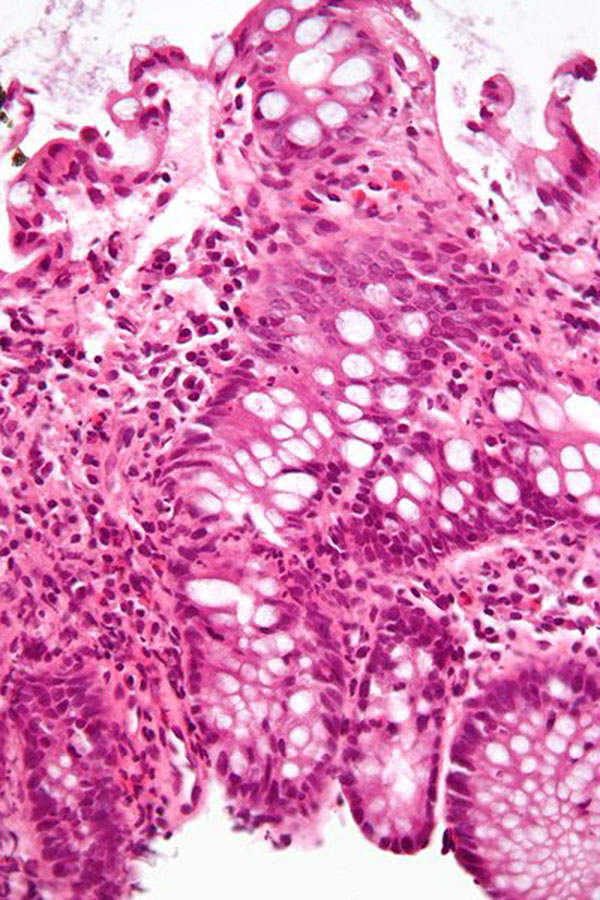
Uceris (budesonide) is glucocorticoid receptor that is indicated for the treatment of ulcerative colitis. It is developed by US-based biopharmaceutical company Santarus, in collaboration with Cosmo Technologies.
Santarus submitted the new drug application (NDA) for uceris to the US Food and Drug Administration (FDA) in December 2011. In January 2013, the company received approval for uceris from the FDA for induction of remission in ulcerative colitis patients.
What is ulcerative colitis?
Ulcerative colitis is a type of chronic inflammatory bowel disease that emanates in the colon (large intestine). It causes inflammation and ulcers inside the lining of colon. The symptoms of the disease include intermittent rectal bleeding, abdominal pain and diarrhoea. The Crohn’s and Colitis Foundation of America estimates that the disease affects about 700,000 people in the US.
Ulceris’s mechanism of action
Uceris contains a glucocorticoid steroid called budesonide. The mechanism of action of the drug includes controlling the rate of protein synthesis, inhibiting the migration of granulocytes and preventing or controlling inflammation.
The drug is available in tablet form in 9mg doses and uses the patented MMX multimatrix system and colonic delivery technology. It contains an enteric coat at the central part for protecting dissolution in gastric juice.
Clinical trials on budesonide
Santarus conducted Phase III clinical trials on uceris between June 2008 and April 2010. It was a randomised, multicentre, double-blind, double-dummy and comparative placebo-controlled study. The study enrolled more than 514 patients. The primary outcome measure of the study was to evaluate the efficacy and safety of 6mg and 9mg doses of uceris for the treatment of patients suffering with active ulcerative colitis.
The secondary outcome measures of the study included a comparison of the 6mg and 9mg doses of uceris with Asacol, plus evaluation of the improvement in rectal bleeding, endoscopic, histological, bio humoral parameters after eight weeks.
Santarus conducted another Phase III clinical study on uceris between June 2008 and June 2010. It was a randomised, double blind, multicentre and parallel assignment study. The study enrolled 510 patients. The primary endpoint of the study was the evaluation of the clinical efficacy and safety of the drug. The secondary outcome measures include examining the efficacy of the drug in treating symptoms, such as rectal bleeding, endoscopic, histological and bio humoral parameters.
The FDA approval for uceris was based on the results of two Phase III clinical trials. They were similarly-designed, open label, randomised, double-blind, placebo-controlled studies. The studies enrolled 970 patients suffering with active, mild to moderate ulcerative colitis. The patients were administered with 6mg and 9mg doses of uceris or placebo. The primary endpoint of the study was induction of remission, which was defined as an Ulcerative Colitis Disease Activity Index (UCDAI) score of <1 after eight weeks.
The results of both the studies were announced in December 2011. The studies demonstrated that the patients administered with uceris 9mg doses were superior to placebo in inducing remission. Uceris was also well tolerated in the patients. The studies also showed that the adverse events encountered during the clinical studies were similar to the placebo.
Santarus has initiated another Phase III clinical trial on uceris in February 2012. It is a multicentre, randomised, double-blind, placebo-controlled study. The study expects to enrol about 500 patients in about 120 clinical sites located across the US, Canada and Europe. The primary outcome measure of the study will include the induction of UCDAI remission in eight weeks. The secondary outcome measures will include finding the induction of clinical remission in ulcerative colitis patients, and time to onset of clinical remission.
Marketing commentary for Santarus’s drug
Santarus plans to launch uceris commercially across the US in March 2013. Other medications available for the treatment of ulcerative colitis include Sulfasalazine (Azulfidine), manufactured by Pfizer, Balsalazide (Colazal) which is produced by Salix Pharmaceuticals, and Olsalazine (Dipentum), manufactured by Alaven Pharmaceuticals.
Related content
Myrbetriq (Mirabegron) – Treatment for Overactive Bladder (OAB)
Myrbetriq (mirabegron) is a beta-3 adrenergic agonist indicated for the treatment of the overactive bladder (OAB) condition.
Omontys (peginesatide) – Treatment for Anaemia
Omontys (peginesatide) is an erythropoiesis-stimulating agent (ESA) developed for the treatment of anaemia associated with chronic kidney disease (CKD).