The product of a joint development programme between Tanox, Genentech and Novartis, Xolair (omalizumab) is a recombinant humanised anti-IgE monoclonal antibody (MAb). It received US Food and Drug Administration approval for the treatment of moderate to severe allergic asthma in adults and adolescents in 2003.
Securing regulatory approval proved a long drawn out process in the US and Europe. European approval was finally secured in October 2005 for the use of Xolair as add-on therapy for control of severe persistent allergic asthma in adults and adolescents.
Its use in Europe is restricted to patients inadequately controlled with inhaled corticosteroids and long-acting ß2-agonists, who also have a positive skin test or in vitro reactivity to a perennial allergen, in addition to reduced lung function, frequent day and night time symptoms and documented severe asthma exacerbations.
In August 2009, Novartis received approval in the EU for Xolair as an add-on therapy for severe persistent allergic asthma in youngsters between the ages of six and 11 years.
The approval was based on a study which showed that Xolair helped in reducing asthma attacks by 50% in one-year-old children. The study also indicated that Xolair demonstrated good overall safety and tolerability in children in the six to 11 age group.
Another study indicated that use of Xolair in children between the ages of six and 11 significantly decreased the need to use oral corticosteroids. The study also showed that the rate of absenteeism among children taking Xolair was lower.
Treatment of allergic asthma
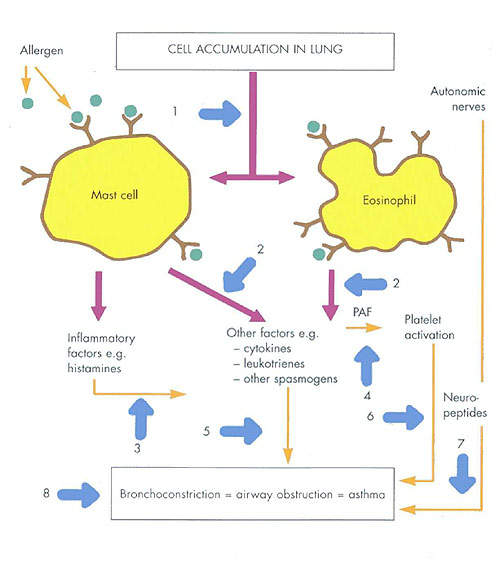
The first in a new class of biological therapies for allergic asthma, Xolair works by binding to free immunoglobulin E (IgE) in the blood so preventing it from binding to mast cells.
This in turn prevents mast cell degranulation and the release of pharmacologically active substances such as histamine and leukotrienes that are the hallmark of the early allergic inflammatory process. By decreasing the amount of IgE bound to mast cells, Xolair disrupts the release of these chemical mediators that cause the clinical symptoms such as inflammation and bronchial constriction.
Xolair differs from most current treatments for asthma in that it addresses the underlying immunopathological processes involved in allergic asthma. Administered by subcutaneous injection, it has only to be given every two to four weeks.
Physicians suggest it will be especially beneficial in:
- Patients who have failed conventional therapies
- Patients who have problems complying with complex treatment regimens
- As add-on therapy to reduce steroid dependence.
Phase III placebo-controlled trials
The efficacy of Xolair has been investigated in a series of clinical trials, which included two pivotal Phase III placebo-controlled trials in over 1,000 allergic asthma patients. Results showed that patients treated with Xolair over a 52-week period had significantly reduced exacerbations of asthma combined with reduced use of steroids and rescue bronchodilators.
In the Xolair treatment arm the average dose of inhaled corticosteroids in the two studies was reduced by 83% and 75% respectively compared with 50% in the placebo groups. Between 40% and 43% of patients on Xolair were able to discontinue steroid use compared with 19% in the placebo groups.
Clinical data supports safety in asthma patients
With any new class of medicines safety and tolerability are of paramount concern. The delays experienced by Xolair in securing regulatory approval were largely due to safety issues.
To meet these concerns additional safety studies were carried out. These included the 900-patient ALTO trial, in which the incidence of serious adverse events was monitored over a 24-week period.
Data from this and other clinical trials indicated that Xolair was generally well tolerated with the frequency of adverse events comparable to control groups. Viral infections, sinusitis, infections of the upper respiratory tract and headache were the most frequently observed adverse events. Serious adverse events were rare and occurred with similar frequency in the Xolair and control treatment arms.
Marketing commentary
Xolair has broken new ground in becoming the first approved biological therapy for the treatment of allergic asthma. This chronic inflammatory disorder of the lungs, in which exposure to allergens triggers an IgE-mediated allergic cascade, affects millions of people and can be fatal. It is now available in most major markets.